Clinical Trial
Audio
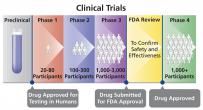
A research study that determines whether a new drug (or other intervention) is both safe and effective for humans. People volunteer to participate in clinical trials (also called interventional studies) to help find better ways to treat, prevent, diagnose, and understand human disease. Clinical trials are conducted in "phases." Results from Phase 1, 2, and 3 trials are used to determine whether a new drug should be approved for sale in the United States. Once a new drug is approved, researchers continue to track its safety in Phase 4 trials.