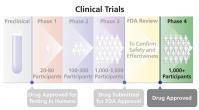
Phase 4 Trial
Audio
Testing in humans that occurs after a drug (or other treatment) has been approved by the U.S. Food and Drug Administration (FDA) and is being marketed for sale. Phase 4 trials are conducted to determine long-term safety and effectiveness and to identify adverse effects that may not have been apparent in prior trials. Phase 4 trials usually include thousands of participants.