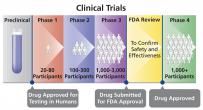
Investigational New Drug Application (IND)
Audio
A drug sponsor’s request to the U.S. Food and Drug Administration (FDA) for approval to test an investigational drug in humans (Phase 1-4 clinical trials). FDA review of an investigational new drug (IND) application ensures that the drug is safe for testing in humans and that testing will not put study participants at unreasonable risk.