Talaromycosis (formerly Penicilliosis)
Epidemiology
Talaromycosis is an invasive fungal infection caused by the dimorphic fungus Talaromyces marneffei (formerly Penicillium marneffei), which is endemic in Southeast Asia (northern Thailand, Vietnam, and Myanmar), East Asia (southern China, Hong Kong, and Taiwan), and South Asia (northeastern India) (see the geographic distribution of talaromycosis in Figure 1)1-4 T. marneffei was formerly classified under the Penicillium subgenus Biverticillium based on morphological characteristics. In 2011, the subgenus Biverticillium was found to form a monophyletic group with Talaromyces that is distinct from Penicillium, and it was taxonomically uniified with the Talaromyces genus.5 Hence, P. marneffei was changed to T. marneffei, and the disease penicilliosis is now called talaromycosis.
HIV is a major risk factor for talaromycosis in highly endemic regions, accounting for approximately 88% of the disease.2 The fungus is also a major cause of HIV-associated opportunistic infections in these regions, making up to 16% of hospital admissions due to AIDS,2,3,6-8 and is a leading cause of HIV-associated bloodstream infections and deaths in Vietnam and southern China.6,9-11 Infection occurs predominantly in individuals2,3,12 who have very advanced HIV disease with a CD4 T lymphocyte (CD4) cell count of <100 cells/mm3. Talaromycosis is increasingly diagnosed in immunocompromised individuals who are returning travelers or immigrants from the endemic regions, and it has been reported in Japan, Australia, Belgium, France, Germany, the Netherlands, Sweden, Switzerland, the United Kingdom, Oman (in the Middle East), and the United States.13,14 Talaromycosis is increasingly recognized in individuals who have a primary immunodeficiency condition (e.g., idiopathic CD4 lymphopenia; anti-interferon-gamma autoantibody-associated immunodeficiency; conditions due to mutations in CYBB or CD40L; or gain-of-function mutation in STAT1/STAT3 pathways) or secondary immunodeficiency conditions (e.g., autoimmune diseases in people on corticosteroids and/or other immunosuppressive therapy; solid and hematological malignancies; solid organ transplantation; hematopoietic stem cell transplantation; and therapy with novel target therapies, such as monoclonal antibodies against CD20 and kinase inhibitors)15 Talaromycosis-related mortality, despite antifungal therapy in people both with and without HIV, is up to 30%.2,3,12,16,17
Similar to other endemic mycoses, talaromycosis is a saprozoonotic infection, meaning the transmissible source has a reservoir both in an abiotic environment and in an animal host. The wild bamboo rat in highland areas in the endemic regions is the known animal reservoir of T. marneffei18,19; however, case-control studies suggest that human infection results from inhalation of fungal spores released from a soil-related environmental reservoir (plants and farmed animals) rather than from direct bamboo rat-to-human transmission.20,21 Talaromycosis incidence increased 30% to 50% during the rainy months in southern Vietnam and northern Thailand,3,22 and was associated with increased humidity and not precipitation,23,24 which suggests that humidity facilitates an expansion of the environmental reservoir, resulting in increased exposure to the fungus. Reactivation of latent infections has been demonstrated in non-autochthonous cases with a history of remote travel to the endemic countries and can occur many years after exposure.13,14,25 One case of presumed laboratory-acquired talaromycosis was reported in an African man with HIV who was at the Pasteur Institute in Paris26; however, laboratory-acquired infection has never been reported from the endemic regions. Donor-acquired transmission has been reported in a lung-transplant recipient from Belgium.27
Clinical Manifestations
Disseminated infection involving multiple organ systems is the most common manifestation of talaromycosis in people with advanced HIV disease. The infection frequently begins as a subacute illness characterized by fever, weight loss, hepatosplenomegaly, lymphadenopathy, and respiratory and gastrointestinal abnormalities.3,28 These clinical features are nonspecific and are indistinguishable from those of disseminated tuberculosis, other systemic mycoses, or infections due to intracellular pathogens such as Salmonella species.
Skin lesions are the most specific but late manifestations of talaromycosis, with central-necrotic papules on the face, trunk, and extremities occurring in 40% to 70% of patients.1,3,29 Pulmonary involvement manifested as cough or shortness of breath occurs in 40% of patients. Gastrointestinal involvement presenting as diarrhea or abdominal pain occurs in 30% of patients. Significant hepatosplenomegaly is present in 70% of patients and together with intra-abdominal lymphadenopathy cause abdominal distention and pain.3,7 Meningoencephalitis is a rare manifestation that occurs in <1% of patients and has a rapid disease course with a mortality of 80%.30 Concurrent infections with other opportunistic pathogens occur in up to 60% of patients, with oropharyngeal candidiasis being the most common.2
Tuberculosis coinfection is common (occurring in up to 22% of patients in highly endemic regions) and complicates disease management because of itraconazole and rifampin drug interactions.3
Common laboratory findings associated with talaromycosis include anemia and thrombocytopenia due to bone marrow infiltration. Anemia can be profound and may require multiple red cell transfusions. Elevation of aminotransferase is common, with a serum aspartate aminotransferase (AST) over alanine aminotransferase (ALT) ratio of approximately 2.3
The median CD4 count in multiple cohorts2,3 is <50 cells/mm3.
The chest radiographical findings are broad, ranging from diffuse interstitial disease to reticulonodular infiltrates to alveola infiltrates causing respiratory failure.31
Diagnosis
A diagnosis of talaromycosis should be considered in all people with HIV with CD4 count <100 cells/mm3 who have traveled to or have lived in talaromycosis-endemic areas and present with a systemic infection involving the reticuloendothelial system (i.e., lymph nodes, liver, spleen, and bone marrow).
Skin lesions in talaromycosis have typical central-necrotic appearance and can be a diagnostic sign. However, skin lesions are a late manifestation of talaromycosis and are absent in up to 60% of patients.1,3,29 The current diagnostic methods for talaromycosis are still based on conventional microscopy, histology, and culture. Culture results usually return within 4 to 5 days but can take up to 28 days. Diagnostic delay, particularly in patients presenting without fever or skin lesions, is associated with increased mortality.2,3,15,32 Antigen detection and polymerase chain reaction (PCR)–based methods are promising rapid diagnostics currently being evaluated.
Microscopy, Histology, and Culture Are the Current Gold Standard Diagnostic Methods
A presumptive diagnosis of talaromycosis can be made based on the microscopic examination of Giemsa-, Wright-, or Gomori Methenamine Silver (GMS)–stained samples of skin lesion scrapings, lymph node aspirate, bone marrow aspirate, or tissue sections showing round-to-oval extracellular and intramacrophage yeast-like organisms measuring 3 to 6 µm in diameter. Identification of a clear midline septum in a dividing yeast cell is what distinguishes T. marneffei from Histoplasma or Candida species.1 In some patients, the fungus can be identified by microscopic examination of a Wright’s-stained peripheral blood smear.33
A definitive diagnosis of talaromycosis can be made by the histopathologic demonstration of the organism in biopsy specimens. There are three histopathological forms. The granulomatous reaction is formed by histiocytes, lymphocytes, and plasma; epithelioid and giant cells and can be seen in reticuloendothelial organs in patients who are HIV-negative or immunocompetent. The suppurative reaction develops with the joining of multiple abscesses seen in the lung and subcutaneous tissues of immunocompetent patients. The anergic and necrotizing reaction is characterized by focal necrosis surrounded by distended histiocytes containing proliferating fungi seen in the lung, liver, and spleen of immunocompromised patients.34
Most frequently, a definitive diagnosis of talaromycosis is based on isolation of the organism from cultures of clinical specimens.
Compared to other endemic dimorphic fungi, T. marneffei grows more readily in standard BACTEC blood culture media and Sabouraud dextrose agar but takes 5 to 14 days to grow and to demonstrate temperature dimorphism. At 25 ºC to 30 ºC, the fungus grows as a mold, producing yellow-green colonies with sulcate folds and a red diffusible pigment in the media. Microscopically, filamentous hyphae with characteristic spore-bearing structures called conidiophores and conidia can be seen. At 32 ºC to 37 ºC, the fungus makes the morphological transition from a mold to a yeast, producing tan-colored colonies without a red diffusible pigment. In laboratory media, only the transitional sausage-shaped cells can be seen microscopically. The round-to-oval yeast cells are only seen in natural tissue.1
Culture yield is the highest from bone marrow (100%), followed by skin lesions (90%) and blood (70%).3,35 Less commonly, talaromycosis has been diagnosed from sputum, pleural fluid, peritoneal fluid, cerebrospinal fluid, pericardium fluid, stool, and urine.
Molecular Diagnosis
Molecular diagnostics for talaromycosis have been based on PCR amplification and sequence identification of specific regions within the fungal ribosome’s internally transcribed spacer regions, the 5.8S rRNA, and the 18S rRNA genes of T. marneffei.36-39 These assays have high specificity (100%), but limited sensitivity (60% to 70%). At present, none of the real-time PCR assays have been prospectively validated, standardized, or commercially developed for clinical use.
Antigen Detection
The commercial assay for the detection of Aspergillus galactomannan cross-reacts with T. marneffei and has a sensitivity of 95.8% (23 of 24 patients with culture-positive talaromycosis were correctly identified) and a specificity of 90.9% (30 of 33 people without talaromycosis were correctly identified) for the detection of talaromycosis (at cutoff index = 1.0).40 However, the galactomannan test also cross reacts with other endemic fungi, such as Histoplasma and Blastomyces, and has not been evaluated prospectively.
The Mp1p enzyme-linked immunosorbent assay (ELISA) has been shown to be more sensitive than blood culture (in 372 culture-proven talaromycosis cases, sensitivity was 86.3% for the Mp1p ELISA and 74% for blood culture) and is highly specific (98.1% specificity in 338 healthy controls and 179 people without HIV but with other infections).41 This assay was used to screen a large serum bank of 8,131 people with HIV in Guangzhou, China, and showed a Mp1p antigenemia prevalence of 9.4%, with prevalence of antigenemia increased from 4.5% to 28.4% as the CD4 count decreased from 200 cells/mm3 to 50 cells/mm3, demonstrating a significant burden of disease in southern China.24 In Vietnam, the Mp1p ELISA identified 4.2% antigenemia in 1,123 asymptomatic people with HIV who had a CD4 count <100 cells/mm3 initiating antiretroviral therapy (ART) in 22 HIV clinics across Vietnam. Antigenemia was found to be independently associated with 12-month mortality.42 These data demonstrate that the Mp1p ELISA has the potential to detect infection earlier than culture allows and can potentially be used as a screening tool for subclinical infection, thereby permitting pre-emptive antifungal therapy to prevent disease development. This is an area of active research.
Matrix-Assisted Laser Desorption/Ionization-Time of Flight Method
The matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) method recently has been used for identification of Talaromyces to the species level from cultured specimens based on either an in-house database generated from an institution’s T. marneffei clinical strain collection43,44 or from the comprehensive National Institutes of Health MDL Mold Library.45 The MALDI-TOF represents a rapid and reliable tool for downstream fungal identification, eliminating the need to demonstrate thermal dimorphism.
Antifungal Susceptibility Testing
The minimum inhibitory concentrations (MICs) have been consistently low for itraconazole, intermediate for amphotericin B, and high for fluconazole. Thus far, only one retrospective case series from Chiang Mai in Thailand correlated MIC data of 30 clinical isolates with patient outcomes. More recent studies reported low MIC values for the newer generation azole drugs voriconazole (MICs 0.016–0.063 µg/mL) and posaconazole (MICs 0.001–0.002 µg/ml), and intermediate to high MIC values of 2 µg/ml to 8 µg/mL for anidulafungin.46 A later study utilized a commercial Sensititre YeastOne YO10 assay.47 These results suggest promising activity of voriconazole and posaconazole for the treatment of talaromycosis and suggest that the echinocandins are less effective against T. marneffei.
Preventing Exposure
Two case-controls studies in Thailand and Vietnam demonstrated that people with World Health Organization Stage 4 HIV disease or a CD4 count <100 cells/mm3 who had an occupational exposure to plants and farmed animals were at increased risk for infection.20,21 The risk was higher in the rainy and humid months.3,22
Residency or a history of traveling to the highland regions (as short as 3 days) was a risk factor for talaromycosis in people with advanced HIV disease in southern Vietnam.20 These data suggest that people with advanced HIV should avoid visiting the areas where talaromycosis is highly endemic, particularly highland regions during the rainy and humid months (BIII).
Preventing Disease
| Preventing First Episode of Talaromycosis (Primary Prophylaxis) |
|---|
Indication for Primary Prophylaxis
Primary Prophylaxis
Indication for Discontinuing Primary Prophylaxis for People Who Reside in Endemic Areas
Indication for Restarting Primary Prophylaxis
|
| Key: ART = antiretroviral therapy; CD4 = CD4 T lymphocyte; PO = orally |
Primary prophylaxis has been shown to reduce the incidence of talaromycosis and other invasive fungal infections. A double-blind, placebo-controlled trial48 in Chiang Mai, Thailand, demonstrated that oral itraconazole 200 mg daily for primary prophylaxis significantly reduced the occurrence of invasive fungal infections (predominantly cryptococcosis and talaromycosis) in people with HIV with a CD4 count <200 cells/mm3.48
In a retrospective study also in Chiang Mai, fluconazole (400 mg weekly) was shown to be as effective as itraconazole (200 mg daily) for primary prophylaxis.49 However, these studies were conducted prior to the widespread use of ART, and had small sample sizes, and a mortality benefit was not observed.
Therefore, primary prophylaxis has not been widely adopted given concerns about long-term toxicity, drug–drug interactions, and costs.
Indication for Primary Prophylaxis
Primary prophylaxis is only recommended for people with HIV with CD4 counts <100 cells/mm3 who reside in the highly endemic regions in northern Thailand, southern China, and northern and southern Vietnam who are unable to have ART for whatever reasons or have treatment failure without access to effective antiretroviral (ARV) options (BI). The drug choices for prophylaxis are oral itraconazole 200 mg once daily (BI) or oral fluconazole 400 mg once weekly (BII).
Primary prophylaxis is not recommended in people with HIV who are on or about to start effective ART and is not recommended in geographic areas outside of the mentioned highly endemic regions (AIII).
For people with HIV who are from the United States and from countries outside of the endemic region who are not on effective ART, have a CD4 count <100 cells/mm3, and must travel to the highly-endemic areas mentioned, primary prophylaxis with either itraconazole or fluconazole should begin 3 days prior to travel to allow serum drug level to reach steady state and may continue for 1 week after travel (BIII).
Discontinuation of Primary Prophylaxis
Primary prophylaxis for talaromycosis can reasonably be discontinued in people with HIV who are ART adherent and have a sustained CD4 count ≥100 cells/mm3 for more than 6 months (BII). In areas where viral load monitoring has replaced CD4 count monitoring, primary prophylaxis can reasonably be discontinued in people with HIV who achieve sustained virologic suppression at least 6 months (BIII).
Treating Disease
| Treating Acute Infection in Severely Ill Patients |
|---|
Preferred Therapy
Alternative Therapy (If Liposomal Amphotericin B Is Not Available)
Alternative Therapy (If Amphotericin B Is Not Available)
Note: Itraconazole is not recommended as induction therapy for talaromycosis (AI). Criteria for Discontinuing Chronic Maintenance Therapy
Criteria for Restarting Chronic Maintenance Therapy
|
| Other Considerations |
|
| Key: ART = antiretroviral therapy; ARV = antiretroviral; CD4 = CD4 T lymphocyte; IV = intravenously; PK = pharmacokinetics; PO = orally; TDM = therapeutic drug monitoring |
Disseminated talaromycosis is fatal if untreated.50
The case fatality rates with antifungal therapy range from 10% to 30%.2,3,6,16
Antifungal therapy for talaromycosis is divided into induction, consolidation, and maintenance phases. The treatment recommendations are based on several observational studies in Thailand and China51-54 and the recent Itraconazole versus Amphotericin B for Penicilliosis (IVAP) randomized, controlled trial in Vietnam.55
In an earlier noncomparative prospective study of 74 patients in Thailand, induction therapy with deoxycholate amphotericin B for 2 weeks followed by consolidation therapy with itraconazole for 10 weeks was shown to be highly effective. Treatment success rate (defined by negative blood culture and resolution of fever and skin lesions at the end of a 12-week treatment course) was 97%.51
Voriconazole has been used for induction therapy in patients who could not tolerate amphotericin B and was shown to have favorable clinical and microbiological outcomes in 8 of 9 patients in Thailand53 and 10 of 14 patients in China.52
The IVAP trial randomized 440 patients across 5 hospitals in Vietnam and demonstrated that induction therapy with amphotericin B was superior to itraconazole with respect to 6-month mortality (absolute risk of death was 11% and 21%, respectively; hazard ratio of death in the itraconazole arm was 1.88 [95% confidence interval, 1.15–3.09, P = 0.012]). Patients in the amphotericin B arm had significantly lower rates of disease complications, including disease relapse and immune reconstitution inflammatory syndrome (IRIS), and had a fourfold faster rate of blood fungal clearance. The difference in mortality between the arms was not dependent on disease severity (based on positive blood culture, blood fungal count, or requirement for oxygen support at presentation) or by a participant’s immune status (CD4 count <50 cells/mm3 or ≥50 cells/mm3), ART status, or intravenous (IV) drug use.55
The recommended induction therapy for all patients, regardless of disease severity, is amphotericin B, preferably liposomal amphotericin B 3 to 5 mg/kg/day where available, or deoxycholate amphotericin B 0.7 mg/kg body weight/day, IV for 2 weeks (AI).
Induction therapy should be followed by consolidation therapy with oral itraconazole, 200 mg every 12 hours for a subsequent duration of 10 weeks (AI).55 After this period, maintenance therapy (or secondary prophylaxis) with oral itraconazole 200 mg/day is recommended to prevent recurrence until the CD4 count rises above 100 cells/mm3 for ≥6 months (AI).56
For patients who are unable to tolerate any form of amphotericin, induction therapy with IV voriconazole 6 mg/kg every 12 hours on Day 1 (loading dose), then 4 mg/kg every 12 hours or with oral voriconazole 600 mg every 12 hours on Day 1 (loading dose), then 400 mg every 12 hours for 2 weeks is recommended (BII).52,53
Thereafter, either oral voriconazole or oral itraconazole 200 mg every 12 hours can be used for consolidation therapy for 10 weeks, followed by itraconazole 200 mg/day for secondary prophylaxis. The optimal dose of voriconazole for secondary prophylaxis beyond 12 weeks has not been studied.
Itraconazole is not recommended as an induction therapy for talaromycosis, regardless of disease severity (AI).55
Special Considerations with Regard to Starting ART
No studies exist regarding the optimal time to start ART in people with HIV who have talaromycosis. In the IVAP trial, the median time to ART initiation, which was similar in both arms, was 3 weeks (range: 1–5 weeks).
Paradoxical IRIS events occurred only in the itraconazole arm (in 11.4% of patients), suggesting that ART can be safely initiated as early as 1 week after starting effective antifungal therapy with amphotericin B (BIII).55
Monitoring of Response to Therapy and Adverse Events (Including IRIS)
Adverse Event Monitoring
Patients treated with amphotericin B should be monitored for infusion-related adverse reactions (fever, rigors, nausea, vomiting), electrolyte disturbances (particularly hypokalemia and hypomagnesemia), nephrotoxicity (rise in creatinine), and anemia. Hydration with 500 mL to 1,000 mL of normal saline and potassium supplementation before each amphotericin B infusion reduces the risk of nephrotoxicity during treatment (AII). Infusion-related adverse reactions can be ameliorated by pre-treatment with acetaminophen and diphenhydramine.
Drug-Drug Interactions and Therapeutic Drug Monitoring
Itraconazole and voriconazole and ARV drugs—such as protease inhibitors (PIs), some integrase strand transfer inhibitors, and non-nucleoside reverse transcriptase inhibitors can have bidirectional interactions with each other, leading to increased or decreased drug concentrations (see Drug–Drug Interactions in the Adult and Adolescent Antiretroviral Guidelines). Close monitoring is recommended when using these drugs together.
In settings where therapeutic drug monitoring (TDM) is available, serum itraconazole and voriconazole levels should be obtained in all patients to ensure adequate drug exposure (BIII). This is because itraconazole and voriconazole can interact with some ARV drugs and absorption of itraconazole can be erratic, and because of the extensive interindividual variability and nonlinear pharmacokinetics of voriconazole. The target serum trough concentration should be >0.5 μg/mL for itraconazole and >1 μg/mL for voriconazole (BIII). Because it is more bioavailable, itraconazole solution is preferred over the capsule formulation.
Prevention and Management of IRIS
Both unmasking and paradoxical IRIS have been described in patients with talaromycosis when ART is initiated.57-59 In the IVAP trial, 188 of 432 (44%) patients had started ART a median of 3 to 4 months before developing talaromycosis, indicating the role of ART in the unmasking of subclinical infection in a significant proportion of patients.55 This finding highlights the need for a sensitive assay to screen for subclinical infection and the importance of pre-emptive antifungal therapy to prevent disease and unmasking IRIS. In patients starting ART after a diagnosis of talaromycosis, paradoxical IRIS events only occurred in patients treated with itraconazole induction therapy,55 demonstrating the role of effective induction therapy with amphotericin B in the prevention of paradoxical IRIS. ART should not be withheld because of concerns for possible development of IRIS (AIII).
Patients with paradoxical IRIS typically present with inflammatory manifestations that include erythematous or immunological skin lesions such as erythema nodosum, as well as large and painful peripheral lymph nodes and synovitis of small joints. Most symptoms can be managed by judicious use of nonsteroid anti-inflammatory medicine. Corticosteroids are reserved for synovitis that interferes with daily function.59 Although the IRIS events in the IVAP trial were not associated with increased mortality and were managed effectively with continuation of ART and antifungal therapy, they were associated with higher morbidity, including lower quality of life and increased diagnostic testing, duration of hospitalization, and cost.55
Managing Treatment Failure and Relapse
Talaromycosis treatment failure and disease relapse were associated with ineffective induction therapy with itraconazole, highlighting the importance of amphotericin B induction therapy.55 On the basis of case series that included very few patients and on clinical experiences, voriconazole is an alternative therapy for patients who are unable to tolerate amphotericin B treatment (BII).
Disease relapse is associated with higher mortality55 and occurs mainly in patients who are not adherent to ART or have virologic failure, as well as in those who are not adherent to itraconazole consolidation or maintenance therapy. Therapy adherence counseling and TDM for itraconazole and voriconazole, if available, are recommended (AIII).
Preventing Recurrence
When to Start Secondary Prophylaxis/Chronic Maintenance Therapy
A study showed that >50% of patients not treated with ART had disease relapse within 6 months after discontinuation of antifungal therapy. A double-blind, placebo-controlled study conducted in Chiang Mai, Thailand, demonstrated that secondary prophylaxis with oral itraconazole 200 mg daily in patients with AIDS reduced the talaromycosis relapse rate from 57% to 0% (p < 0.001).56 All patients who successfully complete induction and consolidation treatment for talaromycosis should receive secondary prophylaxis (maintenance therapy) with oral itraconazole 200 mg/day until they reach criteria for stopping secondary prophylaxis (AI).
When to Stop Secondary Prophylaxis/Chronic Maintenance Therapy
No randomized, controlled study has demonstrated the safety of discontinuation of secondary prophylaxis for talaromycosis. However, a retrospective cohort study60 reported no relapse of talaromycosis after itraconazole was discontinued in patients receiving ART whose CD4 counts were >100 cells/mm3.60
Therefore, secondary prophylaxis for talaromycosis can be discontinued in patients who are ART adherent and have CD4 counts >100 cells/mm3 for at least 6 months (BII).
Secondary prophylaxis can reasonably be discontinued in patients with sustained virologic suppression for ≥6 months (BIII).
Secondary prophylaxis/chronic maintenance therapy should be reintroduced if the CD4 count decreases to <100 cells/mm3 (BIII).
Special Considerations During Pregnancy
The diagnosis and treatment of talaromycosis during pregnancy is similar to that in nonpregnant adults, with the following considerations regarding antifungal use in pregnancy. Amphotericin B has not been shown to be teratogenic in animals, and no increase in fetal anomalies has been seen with its use in humans. Neonates born to people on chronic amphotericin B at delivery should be evaluated for renal dysfunction and hypokalemia.
Itraconazole at high doses has been shown to be teratogenic in animals, but because humans lack the metabolic mechanism accounting for these defects, the animal teratogenicity data are not applicable to humans. Case series in humans do not suggest an increased risk of birth defects with itraconazole, but experience is very limited.61
Voriconazole is Food and Drug Administration Category D because of teratogenicity (cleft palate and renal defects) seen in rats and embryotoxicity in rabbits. No human data on use of voriconazole are available, so use in the first trimester is not recommended.
Substitution of amphotericin B for high-dose azoles in the first trimester is recommended (BIII). People on secondary prophylaxis with itraconazole or other azoles should postpone pregnancy until their CD4 counts have been restored with ART, such that prophylaxis can be discontinued (BIII). If a person becomes pregnant while receiving itraconazole prophylaxis, the decision as to whether to continue should be individualized based on current CD4 count and viral suppression and patient preference.
Figure 1. Geographic Distribution of Talaromycosis
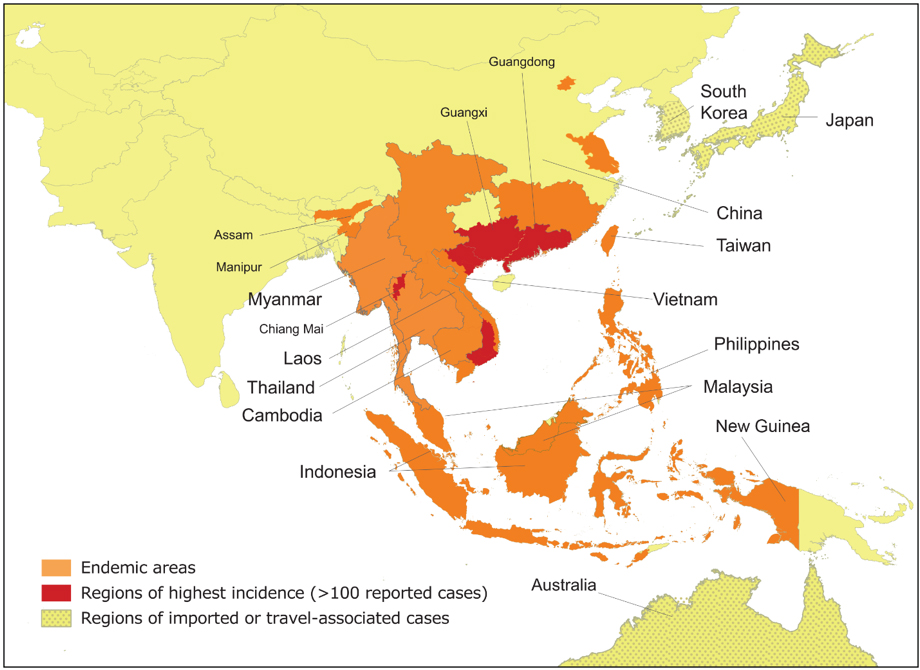
Figure courtesy of Dr. Thuy Le, Division of Infectious Diseases and International Health, Duke University School of Medicine.
References
- Vanittanakom N, Cooper CR, Jr., Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95-110. Available at: https://www.ncbi.nlm.nih.gov/pubmed/16418525.
- Hu Y, Zhang J, Li X, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175(1-2):57-67. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22983901.
- Le T, Wolbers M, Chi NH, et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis. 2011;52(7):945-952. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21427403.
- Ranjana KH, Priyokumar K, Singh TJ, et al. Disseminated Penicillium marneffei infection among HIV-infected patients in Manipur state, India. J Infect. 2002;45(4):268-271. Available at: https://www.ncbi.nlm.nih.gov/pubmed/12423616.
- Samson RA, Yilmaz N, Houbraken J, et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70(1):159-183. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22308048.
- Jiang J, Meng S, Huang S, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect. 2019;25(2):233-241. Available at: https://www.ncbi.nlm.nih.gov/pubmed/29698815.
- Larsson M, Nguyen LH, Wertheim HF, et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther. 2012;9(1):24. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22897817.
- Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008;14(2):103-109. Available at: https://www.ncbi.nlm.nih.gov/pubmed/18382016.
- Feng RF, Ma Y, Liu ZF, et al. [Specific causes of death among 381 AIDS patients who died in hospitals]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34(12):1237-1241. Available at: https://www.ncbi.nlm.nih.gov/pubmed/24518028.
- Nga TV, Parry CM, Le T, et al. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern Vietnam. Trans R Soc Trop Med Hyg. 2012;106(1):26-34. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22137537.
- Qi T, Zhang R, Shen Y, et al. Etiology and clinical features of 229 cases of bloodstream infection among Chinese HIV/AIDS patients: a retrospective cross-sectional study. Eur J Clin Microbiol Infect Dis. 2016;35(11):1767-1770. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27502930.
- Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994;344(8915):110-113. Available at: https://www.ncbi.nlm.nih.gov/pubmed/7912350.
- Antinori S, Gianelli E, Bonaccorso C, et al. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J Travel Med. 2006;13(3):181-188. Available at: https://www.ncbi.nlm.nih.gov/pubmed/16706952.
- Cristofaro P, Mileno MD. Penicillium marneffei infection in HIV-infected travelers. AIDS Alert. 2006;21(12):140-142. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17191362.
- Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5:e19. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26956447.
- Son VT, Khue PM, Strobel M. Penicilliosis and AIDS in Haiphong, Vietnam: evolution and predictive factors of death. Med Mal Infect. 2014;44(11-12):495-501. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25391487.
- Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13:464. Available at: https://www.ncbi.nlm.nih.gov/pubmed/24094273.
- Cao C, Liang L, Wang W, et al. Common reservoirs for Penicillium marneffei infection in humans and rodents, China. Emerg Infect Dis. 2011;17(2):209-214. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21291590.
- Huang X, He G, Lu S, Liang Y, Xi L. Role of Rhizomys pruinosus as a natural animal host of Penicillium marneffei in Guangdong, China. Microb Biotechnol. 2015;8(4):659-664. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25824250.
- Le T, Jonat B, Kim Cuc N, al E. The exposure and geospatial risk factors for AIDS-associated penicilliosis in Vietnam. Presented at: Conference on Retroviruses and Opportunistic Infections; 2015; Seattle, WA.
- Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Praparattanapan J, Nelson KE. Case-control study of risk factors for Penicillium marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin Infect Dis. 1997;24(6):1080-1086. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9195061.
- Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Nelson KE. Seasonal variation of disseminated Penicillium marneffei infections in northern Thailand: a clue to the reservoir? J Infect Dis. 1996;173(6):1490-1493. Available at: https://www.ncbi.nlm.nih.gov/pubmed/8648227.
- Bulterys PL, Le T, Quang VM, Nelson KE, Lloyd-Smith JO. Environmental predictors and incubation period of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis. 2013;56(9):1273-1279. Available at: https://www.ncbi.nlm.nih.gov/pubmed/23386634.
- Wang YF, Xu HF, Han ZG, et al. Serological surveillance for Penicillium marneffei infection in HIV-infected patients during 2004-2011 in Guangzhou, China. Clin Microbiol Infect. 2015;21(5):484-489. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25677258.
- Castro-Lainez MT, Sierra-Hoffman M, J LL-Z, et al. Talaromyces marneffei infection in a non-HIV non-endemic population. IDCases. 2018;12:21-24. Available at: https://www.ncbi.nlm.nih.gov/pubmed/29942740.
- Hilmarsdottir I, Coutellier A, Elbaz J, et al. A French case of laboratory-acquired disseminated Penicillium marneffei infection in a patient with AIDS. Clin Infect Dis. 1994;19(2):357-358. Available at: https://www.ncbi.nlm.nih.gov/pubmed/7986922.
- Hermans F, Ombelet S, Degezelle K, et al. First-in-man observation of Talaromyces marneffei-transmission by organ transplantation. Mycoses. 2017;60(3):213-217. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27687582.
- Sirisanthana T. Penicillium marneffei infection in patients with AIDS. Emerg Infect Dis. 2001;7(3 Suppl):561. Available at: https://www.ncbi.nlm.nih.gov/pubmed/11485672.
- Chen J, Zhang R, Shen Y, et al. Clinical Characteristics and Prognosis of Penicilliosis Among Human Immunodeficiency Virus-Infected Patients in Eastern China. Am J Trop Med Hyg. 2017;96(6):1350-1354. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28719279.
- Le T, Huu Chi N, Kim Cuc NT, et al. AIDS-associated Penicillium marneffei infection of the central nervous system. Clin Infect Dis. 2010;51(12):1458-1462. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21054180.
- Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis. 2017;17(11):e334-e343. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28774701.
- Zheng J, Gui X, Cao Q, et al. A Clinical Study of Acquired Immunodeficiency Syndrome Associated Penicillium Marneffei Infection from a Non-Endemic Area in China. PLoS One. 2015;10(6):e0130376. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26083736.
- Supparatpinyo K, Sirisanthana T. Disseminated Penicillium marneffei infection diagnosed on examination of a peripheral blood smear of a patient with human immunodeficiency virus infection. Clin Infect Dis. 1994;18(2):246-247. Available at: https://www.ncbi.nlm.nih.gov/pubmed/8161635.
- Deng Z, Ribas JL, Gibson DW, Connor DH. Infections caused by Penicillium marneffei in China and Southeast Asia: review of eighteen published cases and report of four more Chinese cases. Rev Infect Dis. 1988;10(3):640-652. Available at: https://www.ncbi.nlm.nih.gov/pubmed/3293165.
- Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson KE, Sirisanthana T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14(4):871-874. Available at: https://www.ncbi.nlm.nih.gov/pubmed/1315586.
- LoBuglio KF, Taylor JW. Phylogeny and PCR identification of the human pathogenic fungus Penicillium marneffei. J Clin Microbiol. 1995;33(1):85-89. Available at: https://www.ncbi.nlm.nih.gov/pubmed/7699073.
- Pornprasert S, Praparattanapan J, Khamwan C, et al. Development of TaqMan real-time polymerase chain reaction for the detection and identification of Penicillium marneffei. Mycoses. 2009;52(6):487-492. Available at: https://www.ncbi.nlm.nih.gov/pubmed/19207847.
- Hien HTA, Thanh TT, Thu NTM, et al. Development and evaluation of a real-time polymerase chain reaction assay for the rapid detection of Talaromyces marneffei MP1 gene in human plasma. Mycoses. 2016;59(12):773-780. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27453379.
- Dankai W, Pongpom M, Vanittanakom N. Validation of reference genes for real-time quantitative RT-PCR studies in Talaromyces marneffei. J Microbiol Methods. 2015;118:42-50. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26327538.
- Huang YT, Hung CC, Liao CH, Sun HY, Chang SC, Chen YC. Detection of circulating galactomannan in serum samples for diagnosis of Penicillium marneffei infection and cryptococcosis among patients infected with human immunodeficiency virus. J Clin Microbiol. 2007;45(9):2858-2862. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17596363.
- Thu NT, Chan JF, Hien HTA, et al. Clinical performance of the Mp1p immunoassay for rapid diagnosis of Talaromyces marneffei infection. Presented at: Conference on Retroviruses and Opportunistic Infections; 2017; Seattle, WA.
- Thu N, Dat V, Chan J, et al. Asymptomatic Talaromyces marneffei infection is associated with HIV mortality. Presented at: Asia Pacific AIDS and CoInfection Conference; 2018; Hong Kong, China.
- Borman AM, Fraser M, Szekely A, Johnson EM. Rapid and robust identification of clinical isolates of Talaromyces marneffei based on MALDI-TOF mass spectrometry or dimorphism in Galleria mellonella. Med Mycol. 2019;57(8):969-975. Available at: https://www.ncbi.nlm.nih.gov/pubmed/30649411.
- Lau SK, Lam CS, Ngan AH, et al. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for rapid identification of mold and yeast cultures of Penicillium marneffei. BMC Microbiol. 2016;16:36. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26965891.
- Li L, Chen K, Dhungana N, Jang Y, Chaturvedi V, Desmond E. Characterization of Clinical Isolates of Talaromyces marneffei and Related Species, California, USA. Emerg Infect Dis. 2019;25(9):1765-1768. Available at: https://www.ncbi.nlm.nih.gov/pubmed/31441765.
- Lau SK, Lo GC, Lam CS, et al. In Vitro Activity of Posaconazole against Talaromyces marneffei by Broth Microdilution and Etest Methods and Comparison to Itraconazole, Voriconazole, and Anidulafungin. Antimicrob Agents Chemother. 2017;61(3). Available at: https://www.ncbi.nlm.nih.gov/pubmed/28031205.
- Lei HL, Li LH, Chen WS, et al. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur J Clin Microbiol Infect Dis. 2018;37(6):1099-1102. Available at: https://www.ncbi.nlm.nih.gov/pubmed/29536323.
- Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin Infect Dis. 2002;34(2):277-284. Available at: https://www.ncbi.nlm.nih.gov/pubmed/11740718.
- Chaiwarith R, Fakthongyoo A, Praparattanapan J, Boonmee D, Sirisanthana T, Supparatpinyo K. Itraconazole vs fluconazole as a primary prophylaxis for fungal infections in HIV-infected patients in Thailand. Curr HIV Res. 2011;9(5):334-338. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21916838.
- Supparatpinyo K, Nelson KE, Merz WG, et al. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob Agents Chemother. 1993;37(11):2407-2411. Available at: https://www.ncbi.nlm.nih.gov/pubmed/8285625.
- Sirisanthana T, Supparatpinyo K, Perriens J, Nelson KE. Amphotericin B and itraconazole for treatment of disseminated Penicillium marneffei infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26(5):1107-1110. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9597237.
- Ouyang Y, Cai S, Liang H, Cao C. Administration of Voriconazole in Disseminated Talaromyces (Penicillium) Marneffei Infection: A Retrospective Study. Mycopathologia. 2017;182(5-6):569-575. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28108867.
- Supparatpinyo K, Schlamm HT. Voriconazole as therapy for systemic Penicillium marneffei infections in AIDS patients. Am J Trop Med Hyg. 2007;77(2):350-353. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17690411.
- Supparatpinyo K, Chiewchanvit S, Hirunsri P, et al. An efficacy study of itraconazole in the treatment of Penicillium marneffei infection. J Med Assoc Thai. 1992;75(12):688-691. Available at: https://www.ncbi.nlm.nih.gov/pubmed/1339213.
- Le T, Kinh NV, Cuc NTK, et al. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N Engl J Med. 2017;376(24):2329-2340. Available at: https://www.ncbi.nlm.nih.gov/pubmed/28614691.
- Supparatpinyo K, Perriens J, Nelson KE, Sirisanthana T. A controlled trial of itraconazole to prevent relapse of Penicillium marneffei infection in patients infected with the human immunodeficiency virus. N Engl J Med. 1998;339(24):1739-1743. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9845708.
- Hall C, Hajjawi R, Barlow G, Thaker H, Adams K, Moss P. Penicillium marneffei presenting as an immune reconstitution inflammatory syndrome (IRIS) in a patient with advanced HIV. BMJ Case Rep. 2013;2013. Available at: https://www.ncbi.nlm.nih.gov/pubmed/23362074.
- Liu X, Wu H, Huang X. Disseminated Penicillium marneffei infection with IRIS. IDCases. 2015;2(4):92-93. Available at: https://www.ncbi.nlm.nih.gov/pubmed/26793468.
- Thanh NT, Vinh LD, Liem NT, et al. Clinical features of three patients with paradoxical immune reconstitution inflammatory syndrome associated with Talaromyces marneffei infection. Med Mycol Case Rep. 2018;19:33-37. Available at: https://www.ncbi.nlm.nih.gov/pubmed/29379703.
- Chaiwarith R, Charoenyos N, Sirisanthana T, Supparatpinyo K. Discontinuation of secondary prophylaxis against penicilliosis marneffei in AIDS patients after HAART. AIDS. 2007;21(3):365-367. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17255744.
- Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O, Charlier C. Antifungal drugs during pregnancy: an updated review. J Antimicrob Chemother. 2015;70(1):14-22. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25204341.
Preventing Disease
| Preventing First Episode of Talaromycosis (Primary Prophylaxis) |
|---|
Indication for Primary Prophylaxis
Primary Prophylaxis
Indication for Discontinuing Primary Prophylaxis for People Who Reside in Endemic Areas
Indication for Restarting Primary Prophylaxis CD4 count decreases to <100 cells/mm3 (BIII) and the person still resides in or travels to high-risk areas. Primary prophylaxis for travelers may begin 3 days prior to travel to allow serum drug level to reach steady state and may continue for 1 week after travel (BIII). |
| Key: ART = antiretroviral therapy; CD4 = CD4 T lymphocyte; PO = orally |
Treating Disease
| Treating Acute Infection in Severely Ill Patients |
|---|
Preferred Therapy
Alternative Therapy (If Liposomal Amphotericin B Is Not Available)
Alternative Therapy (If Amphotericin B Is Not Available)
Note: Itraconazole is not recommended as induction therapy for talaromycosis (AI). Criteria for Discontinuing Chronic Maintenance Therapy
Criteria for Restarting Chronic Maintenance Therapy
|
| Other Considerations |
Substitution of amphotericin B for high-dose azoles in the first trimester is recommended (BIII). People on secondary prophylaxis with itraconazole or other azoles should postpone pregnancy until their CD4 counts have been restored with ART, such that prophylaxis can be discontinued (BIII). |
| Key: ART = antiretroviral therapy; ARV = antiretroviral; CD4 = CD4 T lymphocyte; IV = intravenously; PK = pharmacokinetics; PO = orally; TDM = therapeutic drug monitoring |
Download Guidelines
- Section Only PDF (248.34 KB)
- Full Guideline PDF (6.08 MB)
- Tables Only PDF (918.31 KB)
