Drug information
| drug-audio-en-Romidepsin.mp3 |
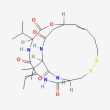
C24 H36 N4 O6 S2
(1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
Romidepsin is in Phase 2 development as a latency-reversing agent for HIV treatment.
(Compound details obtained from PubChem,1 Treatment Action Group website,2 and Journal of Biomedicine and Biotechnology article3)
Pharmacology
Mechanism of Action
Latency-reversing agent, specifically a histone deacetylase inhibitor (HDACi).2 Romidepsin, a bicyclic depsipeptide, is an HDACi targeting the Class I HDAC enzymes HDAC1 and HDAC2.4–6 Romidepsin is an FDA-approved drug indicated for the treatment of cutaneous T-cell lymphoma. As an HIV therapeutic, romidepsin is currently being investigated as an agent for reactivating latent HIV expression.2 In HIV-1 latency, HDACs are recruited to the proviral 5' long terminal repeat (LTR), where they catalyze deacetylation of lysine residues on histones, resulting in chromatin condensation on nucleosome 1 (nuc-1) and preventing HIV transcription. Inhibition of HDAC activity promotes histone acetylation (hyperacetylation) of lysine residues by histone acetyltransferases (HATs), leading to chromatin relaxation and transcriptional activation.6,7 Some research suggests that the activity of HDACis in inducing HIV transcription may not be caused by direct effects on histone acetylation, but may be caused by effects on other non-histone proteins.5,8
Half-life (T½)
In a study of participants with T-cell lymphomas who were receiving intravenous (IV) romidepsin 14 mg/m2 over 4 hours on days 1, 8, and 15 of a 28-day cycle, the terminal half-life of romidepsin was approximately 3 hours.4
Metabolism/Elimination
In vitro, romidepsin is extensively metabolized. The primary metabolic enzyme is CYP3A4. The CYP3A5, CYP1A1, CYP2B6, and CYP2C19 enzymes also contribute to romidepsin metabolism, but to a lesser extent. Therapeutic concentrations of romidepsin used to treat T-cell lymphomas did not competitively inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4 and did not cause significant induction of CYP1A2, CYP2B6, and CYP3A4 in vitro.4
Select Clinical Trials
Study Identifiers: REDUC; NCT02092116
Sponsor: Bionor Immuno AS
Phase: 1b/2a
Status: This study has been completed.
Study Purpose: The REDUC trial was a two-part open-label study. The purpose of Part A was to verify a safe and effective dose of romidepsin for latency reversal prior to use in the second part of the study. The purpose of Part B was to evaluate the effect of the therapeutic HIV vaccine Vacc-4x plus adjuvant combined with romidepsin on the latent HIV reservoir and on viral load control during an analytical treatment interruption of ART.
Study Population:
- Participants were adults with HIV who were on ART at the time of enrollment and who had been on ART for at least 1 year.
- Participants had HIV-1 RNA <50 copies/mL for at least 1 year and had CD4 counts ≥500 cells/mm3. Participants’ nadir CD4 counts in the past 2 years were ≥200 cells/mm3.9–11
Selected Study Results: Results from Part A of the trial published in PLoS Pathogens (2015) showed that romidepsin could safely reverse HIV latency. Part B results published in Lancet HIV (2016) indicated that the combination of Vacc-4x plus adjuvant and romidepsin produced approximately a 40% reduction in the size of the latent HIV reservoir from baseline to follow-up. The combined strategy, however, had no effect on prolonging the time from ART interruption to viral rebound.10,11
Study Identifiers: ACTG 5315; NCT01933594
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Phase: 1/2
Status: This study has been completed.
Study Purpose: The purpose of this study was to evaluate the safety of single and multiple doses of romidepsin and the effectiveness of single and multiple doses of romidepsin in inducing HIV-1 expression in latently infected CD4 T cells.
Study Population:
- Participants were adults with HIV who were on an ART regimen containing two or more NRTIs in combination with raltegravir, dolutegravir, or efavirenz for at least the past 90 days prior to study entry.
- Participants had HIV RNA <50 copies/mL for at least the past 365 days prior to study entry.
- Participants had CD4 counts ≥300 cells/mm3 prior to study entry.12
Selected Study Results: Results published in The Journal of Infectious Diseases (2021) indicated that single and multiple romidepsin infusions were generally safe, with no Grade 3 or higher adverse events (AEs) reported in the single-dose cohorts and one case of Grade 3 neutropenia (possibly related to romidepsin) reported in the multidose cohort. Both single and multiple romidepsin administration had no significant effect on reactivating latent HIV in participants on suppressive ART.13
Study Identifiers: ROADMAP; NCT02850016
Sponsor: Rockefeller University
Phase: 2a
Status: This study has been completed.
Study Purpose: The purpose of this open-label study was to compare the efficacy of romidepsin plus the investigational bNAb 3BNC117 to the efficacy of romidepsin administered alone on delaying or preventing viral rebound during an analytical treatment interruption of ART.
Study Population:
- Participants were adults with HIV who were receiving ART for at least 18 months.
- Participants on a PI- or NNRTI-based ART regimen or on a cobicistat-containing regimen were willing to switch to an INI-based regimen prior to enrollment.
- Participants had HIV RNA <50 copies/mL for at least 12 months and had CD4 counts >500 cells/mm3 at screening.14,15
Selected Study Results: Results published in The Lancet Microbe (2022) showed that romidepsin plus 3BNC117 and romidepsin administered alone had no substantial effect on reducing latent HIV reservoir size in participants on suppressive ART. Additionally, romidepsin plus 3BNC117, compared to romidepsin administered alone, was not effective in delaying the time to viral rebound during analytical treatment interruption, The observed time to viral rebound was not clinically meaningful in either group.15
Study Identifiers: BIOSKILL; EudraCT 2015-003186-28
Sponsor: Bionor Pharma ASA
Phase: 2
Status: This study has been completed.
Study Purpose: The purpose of this study was to evaluate the safety and efficacy of Vacc-4x plus adjuvant when given prior to romidepsin. Effects on viral load, latent HIV reservoir size, and immune responses were measured.
Study Population:
- Participants were adults with HIV who had been receiving uninterrupted ART for at least the past 3 years.
- Participants had sustained HIV RNA <20 copies/mL and CD4 counts ≥500 cells/mm3 at screening.
- Participants had nadir CD4 counts ≥250 cells/mm3.16,17
Study Identifiers: eCLEAR; NCT03041012
Sponsor: Aarhus University Hospital
Phase: 1b/2a
Status: This study has been completed.
Study Purpose: The purpose of this open-label study was to evaluate whether the early administration of romidepsin and/or 3BNC117 in treatment-naive individuals who were initiating ART could reduce the time to viral suppression, limit the size of the latent HIV reservoir, and delay the time to viral rebound during an analytical treatment interruption of ART.
Study Population:
- Participants were newly diagnosed treatment-naive adults with HIV.
- Participants had CD4 counts >200 cells/mm3 at screening.2,18,19
Selected Study Results: Results presented at CROI 2022 and published in Nature Medicine (2022) showed that the early administration of 3BNC117, with or without romidepsin, led to faster viral load decline and significantly enhanced the elimination of infected cells after ART initiation, as compared to what was observed in participants receiving ART only. After 1 year, latent HIV reservoir size was reduced from baseline in all treatment groups, with the greatest reduction seen in those receiving 3BNC117 plus ART. Among participants who received 3BNC117 and had 3BNC117-sensitive virus, 80% maintained viral control during treatment interruption of ART. In contrast, among participants who had 3BNC117-resistant virus or did not receive 3BNC117, only 20% maintained viral control during treatment interruption.19,20
Additional early-phase studies investigating romidepsin for HIV treatment have been or are being conducted, including:
- BCN02-Romi (NCT02616874): A Phase 1 study that enrolled participants from the BCN01 vaccine trial in which two viral vector-based therapeutic HIV vaccines (ChAdV63.HIVconsv and MVA.HIVconsv prime/boost regimen) were evaluated. In the BCN02-Romi trial, participants were given booster immunizations with MVA.HIVconsv in combination with romidepsin and underwent an analytical treatment interruption of ART. BCN02-Romi was completed, and results are available from Frontiers in Immunology (2020).21,22
- SYNACTHIV (NCT05230368): A Phase 1 trial evaluating the safety and tolerability of a combination of two HIV latency-reversing agents (decitabine and romidepsin) in men with subtype B HIV who have viral suppression on ART. This study is currently recruiting participants.23
Adverse Events
REDUC (NCT02092116)
In Part A of this Phase 1b/2a trial, no severe AEs or suspected unexpected serious adverse reactions (SUSARs) occurred in the six participants enrolled in the study. Among 41 reported AEs, 35 were considered related to romidepsin. All romidepsin-related AEs were Grade 1 and resolved within a few days. The most common romidepsin-related AEs were abdominal symptoms (such as nausea, borborygmi, abdominal pain) and fatigue. Changes in white blood cell counts and T cell counts that occurred during the study were considered modest. After the second romidepsin infusion, the lowest counts were seen, and after the third infusion, no further decline in counts occurred. The following were not observed: neutrophil counts below 1000 cells/μL, CD4 T cell counts below 350 cells/μL, or platelet counts below 100,000 cells/μL.10
In Part B of the REDUC trial, 20 participants were enrolled. Only one of three participants who discontinued the study during the immunization phase dropped out because of AEs that were potentially related to Vacc-4x. One hundred forty-one total AEs were reported. Of the drug-related AEs, 42 (31%) were Grade 1 AEs related to Vacc-4x and adjuvant, one was a Grade 2 AE related to Vacc-4x and adjuvant, and 57 (40%) were Grade 1 AEs related to romidepsin. The most common AEs associated with Vacc-4x and adjuvant were transient redness and itching at the injection site. Fatigue and nausea were the most frequently reported AEs associated with romidepsin. Only one romidepsin-related AE, Grade 1 hair loss, was not resolved at the end of the study. During the treatment interruption, six Grade 1 AEs and three Grade 2-3 AEs were reported. Four of the Grade 1 events were considered related to the treatment interruption. No drug-related serious adverse events (SAEs) occurred during the study.11
ACTG 5315 (NCT01933594)
In this Phase 1/2 study, 43 participants were enrolled in one of the three single-dose cohorts and received one infusion of either romidepsin (n = 36) or placebo (n = 7). Romidepsin was reported as being well-tolerated by participants at all dose levels. No treatment-related Grade 3 AEs occurred. Seven participants receiving romidepsin experienced Grade 2 AEs that were possibly treatment-related, including fatigue, increased QTc interval, hypophosphatemia, and neutropenia.13
Among 16 total participants enrolled to receive multiple-dose romidepsin (n = 13) or placebo (n = 3), one participant experienced Grade 3 neutropenia that was deemed to be possibly treatment related. Four participants experienced one or more Grade 2 AEs that were considered possibly, probably, or definitely romidepsin-related – blurred vision, neutropenia, nausea, fatigue, and headache.13
ROADMAP (NCT02850016)
In this Phase 2a trial, 11 participants received romidepsin plus 3BNC117 and nine participants received romidepsin alone. All participants in both groups experienced AEs, most of which were mild to moderate in severity. Out of 267 reported AEs, 39 were considered drug-related and 159 were deemed at least possibly related to study treatments. Two SAEs occurred in the romidepsin group, one of which was related to romidepsin and resolved without intervention – increased direct bilirubin. The most common AEs associated with romidepsin were nausea, headache, chills, and vomiting. More drug-related AEs occurred with romidepsin than with 3BNC117. Transient QTc interval prolongation without associated clinical symptoms was seen in three participants one day after romidepsin infusion.15
eCLEAR (NCT03041012)
In the Phase 1b/2a eCLEAR study, treatment-naive participants received ART only (n = 15); ART plus 3BNC117 (n = 14); ART plus romidepsin (n = 12); or a combination of ART, 3BNC117, and romidepsin (n = 14). Out of 319 reported AEs, 205 were considered unrelated to study treatment. Twenty-nine AEs, most of which were mild, were related to 3BNC117. The most common 3BNC117-related AEs were fatigue and headache. Eighty-five AEs were related to romidepsin, of which 13 were Grade 2 in intensity. The most common romidepsin-related AEs were nausea and fatigue. Six SAEs were reported, but none were related to 3BNC117 or romidepsin.19
Additional AEs known to be associated with romidepsin are described in the FDA-approved Full Prescribing Information for Istodax.4
Drug Interactions
Studies have indicated that therapeutic concentrations of romidepsin used to treat T-cell lymphomas did not competitively inhibit the following CYP enzymes in vitro: CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A4. Also, romidepsin did not cause any significant induction of CYP1A2, CYP2B6, and CYP3A4 in vitro. Therefore, when CYP substrates are coadministered with romidepsin, drug-drug interactions resulting from CYP induction or inhibition by romidepsin are not expected to occur.4
Romidepsin is extensively metabolized by CYP3A4, and strong CYP3A4 inhibitors can increase romidepsin concentrations. Initial coadministration of romidepsin with strong CYP3A4 inhibitors should include monitoring for toxicities associated with increased romidepsin exposure. A drug interaction study with coadministered rifampin (a strong CYP3A4 inducer) found that romidepsin exposure was increased significantly; therefore, coadministration of romidepsin with rifampin should be avoided. Coadministration of romidepsin with other potent CYP3A4 inducers should be avoided.4
Romidepsin is a substrate of P-gp, and coadministration of romidepsin with drugs that inhibit P-gp may increase concentrations of romidepsin.4
Additional known interactions between romidepsin and coadministered drugs are described in the FDA-approved Full Prescribing Information for Istodax.4
References
- National Center for Biotechnology Information. PubChem compound summary for CID 3425, romidepsin. Accessed March 28, 2024
- Treatment Action Group website. Research toward a cure trials. Accessed March 28, 2024
- Masetti R, Serravalle S, Biagi C, Pession A. The role of HDACs inhibitors in childhood and adolescence acute leukemias. J Biomed Biotechnol. Published online Article ID 148046 2011. doi:10.1155/2011/148046. Accessed March 28, 2024
- Celgene Corporation. Istodax: full prescribing information, January 31. 2023. DailyMed. Accessed March 28, 2024
- Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 2013;21(6):277-285. doi:10.1016/j.tim.2013.02.005. Accessed March 28, 2024
- Rasmussen TA, Tolstrup M, Winckelmann A, Østergaard L, Søgaard OS. Eliminating the latent HIV reservoir by reactivation strategies. Hum Vaccines Immunother. 2013;9(4):790-799. Accessed March 28, 2024
- Matalon S, Rasmussen TA, Dinarello CA. Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med. 2011;17(5-6):466-472. doi:10.2119/molmed.2011.00076. Accessed March 28, 2024
- Elliott JH, Wightman F, Solomon A, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;13(10(11)):e1004473. doi: 10.1371/journal.ppat.1004473. doi:10.1371/journal.ppat.1004473. Accessed March 28, 2024
- Bionor Immuno AS. An open Phase I/IIa study to evaluate the safety and effect of therapeutic HIV-1 immunization using Vacc-4x + rhuGM-CSF, and HIV-1 reactivation using romidepsin, on the viral reservoir in virologically suppressed HIV-1 infected adults on cART. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on March 3, 2014. NLM Identifier: NCT02092116. Accessed March 28, 2024
- Søgaard OS, Graversen ME, Leth S, et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 2015;11(9). doi:10.1371/journal.ppat.1005142. Accessed March 28, 2024
- Leth S, Schleimann MH, Nissen SK, et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3(10):e463-e472. doi:10.1016/S2352-3018(16)30055-8. Accessed March 28, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). A Phase I/II study of romidepsin in HIV-infected adults with suppressed viremia on antiretroviral therapy to assess safety, tolerability, and activation of HIV-1 expression. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on August 28, 2013. NLM Identifier: NCT01933594. Accessed March 28, 2024
- McMahon DK, Zheng L, Cyktor JC, et al. A Phase 1/2 randomized, placebo-controlled trial of romidespin in persons with HIV-1 on suppressive antiretroviral therapy. J Infect Dis. 2020;224(4):648-656. doi:10.1093/infdis/jiaa777. Accessed March 28, 2024
- Rockefeller University. A Phase 2a, randomized study of romidepsin with or without 3BNC117 to evaluate the effects on the HIV-1 reservoir (ROADMAP). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 26, 2016. NLM Identifier: NCT02850016. Accessed March 28, 2024
- Gruell H, Gunst JD, Cohen YZ, et al. Effect of 3BNC117 and romidepsin on the HIV-1 reservoir in people taking suppressive antiretroviral therapy (ROADMAP): a randomised, open-label, phase 2A trial. Lancet Microbe. 2022;3(3):e203-e214. doi:10.1016/S2666-5247(21)00239-1. Accessed March 28, 2024
- EU Clinical Trials Register. EudraCT Number: 2015-003186-28; BIOSKILL: studying Vacc-4x, an HIV therapeutic vaccine, an assessment of immune-mediated anti-viral effects, when administered with adjuvant GM-CSF prior to HIV latent reservoir activation by the HDAC inhibitor, romidepsin. Accessed March 28, 2024
- Bionor Pharma: Press release, dated December 21, 2015. Bionor announces that the HIV ’Shock & Kill’ trial REDUC with Vacc-4x and romidepsin meets its primary endpoint by significantly reducing latent HIV reservoir and demonstrates control of viral load. Accessed March 28, 2024
- Aarhus University Hospital. Early administration of latency reversing therapy and broadly neutralizing antibodies to limit the establishment of the HIV-1 reservoir during initiation of antiretroviral treatment - a randomized controlled trial. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 20, 2017. NLM Identifier: NCT03041012. Accessed March 28, 2024
- Gunst JD, Pahus MH, Rosás-Umbert M, et al. Early intervention with 3BNC117 and romidepsin at antiretroviral treatment initiation in people with HIV-1: a phase 1b/2a, randomized trial. Nat Med. 2022;28(11):2424-2435. doi:10.1038/s41591-022-02023-7. Accessed March 28, 2024
- Gunst JD, Pahus MH, Rosás-Umbert M, et al. The impact of 3BNC117 and romidepsin treatment at ART initiation on HIV-1 persistence. International AIDS Society (IAS) Conference on Retroviruses and Opportunistic Infections (CROI); February 12-16, 2022; Virtual. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2022. Accessed March 28, 2024
- IrsiCaixa. An open label Phase I trial to evaluate the safety and effect of HIVconsv vaccines in combination with histone deacetylase inhibitor romidepsin on the viral rebound kinetic after treatment interruption in early treated HIV-1 infected individuals (BCN02-Romi). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered November 9, 2015. NLM Identifier: NCT02616874. Accessed March 28, 2024
- Mothe B, Rosás-Umbert M, Coll P, et al. HIVconsv vaccines and romidepsin in early-treated HIV-1-infected individuals: safety, immunogenicity and effect on the viral reservoir (Study BCN02). Front Immunol. 2020;11:823. doi:10.3389/fimmu.2020.00823. Accessed March 28, 2024
- ANRS, Emerging Infectious Diseases. A pilot open label Phase I trial to evaluate the safety and the tolerability of a combination of two HIV-1 inducers in HIV+ sub-type B patients under cART with undetectable viral load. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on December 23, 2021. NLM Identifier: NCT05230368. Accessed March 28, 2024
Last Reviewed: March 28, 2024