New Drug Application (NDA)
Audio
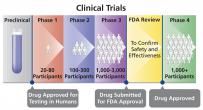
A drug sponsor's request to the U.S. Food and Drug Administration (FDA) for approval to sell and market a new drug in the United States. A new drug application (NDA) includes enough information for the FDA to determine whether the new drug is safe and effective; whether the drug’s benefits outweigh its risks; whether the proposed drug label (package insert) is appropriate; and whether the drug manufacturing standards are adequate. Information included in an NDA is based on laboratory and animal preclinical studies and testing in humans (Phase 1-4 clinical trials).