Drug information
| drug-audio-en-Tenofovir-Based Microbicides.mp3 |
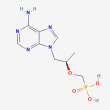
C9 H14 N5 O4 P
[(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid
Tenofovir vaginal gel has been studied in a Phase 3 trial, but it is no longer under development. Tenofovir rectal gel has been studied in a Phase 2 trial. Intravaginal rings containing tenofovir are in Phase 2a development. Other tenofovir-based microbicides are in earlier phases of study.
(Compound details obtained from PubChem,1 NIAID Therapeutics Database,2 Antiviral Therapy article,3 and ClinicalTrials.gov4-12)
Pharmacology
Mechanism of Action
Microbicide; nucleoside reverse transcriptase inhibitor (NRTI). HIV-specific topical microbicides formulated with ARV drugs, such as tenofovir, are being developed as a pre-exposure prophylaxis (PrEP) strategy to prevent the sexual transmission of HIV. ARV-based topical microbicides are designed to inhibit the infection process at the vaginal or rectal mucosa and directly interfere with the HIV replication cycle.13-16
Tenofovir, an adenosine nucleoside monophosphate (nucleotide) analog, is intracellularly phosphorylated to the active metabolite tenofovir diphosphate (TFV-DP). TFV-DP competitively inhibits the activity of HIV reverse transcriptase and causes DNA chain termination.15,17 Tenofovir, formulated as a microbicide, has primarily been studied for preventing sexually transmitted HIV; secondarily, it has been studied for its potential impact on preventing other sexually transmitted infections, such as HSV-2.4
Several different tenofovir-based microbicide products have been studied in clinical trials, including vaginal dosage forms (gel, film, tablet, ring, and insert), as well as rectal dosage forms (gel, douche, and insert).4-10,18,19 The tenofovir vaginal gel was the furthest along in development, with a completed Phase 3 efficacy study (FACTS 001; NCT01386294). However, results from this trial found that the gel did not protect women from acquiring HIV and that product adherence was an important factor in the overall results of the trial.4,20 Currently, the tenofovir rectal gel and intravaginal rings (IVRs) containing tenofovir are the most advanced products in Phase 2 development.5,8
Half-life (T½)
In a study of tenofovir vaginal gel in macaques, the estimated intracellular half-life of tenofovir in vaginal lymphocytes was 25 hours. In comparison, with orally dosed tenofovir DF in macaques, the tenofovir intracellular half-life in PBMCs was 49 hours.21
In a study that compared rectally applied tenofovir gel to oral tenofovir DF in healthy adults without HIV, the tenofovir plasma half-life was approximately 4.6 hours with single rectal dosing, 6.6 hours with multiple rectal dosing, and 11 hours with single oral dosing.22
Metabolism/Elimination
Tenofovir is not a CYP substrate. Approximately 70% to 80% of an intravenously administered dose of tenofovir is recovered in the urine as unchanged drug.15
Of note, in a study assessing the impact of vaginal bacteria on tenofovir gel efficacy in women participating in the CAPRISA 004 trial, researchers found that tenofovir gel was substantially less effective in women who had non-Lactobacillus bacteria than in women who had Lactobacillus-dominant bacteria. Gardnerella vaginalis and other anaerobes rapidly metabolized and depleted tenofovir before the drug could be changed to its active form in target cells.23
Resistance
In the CAPRISA 004 trial (NCT00441298), which evaluated tenofovir 1% vaginal gel, no resistance mutations related to tenofovir, thymidine analog mutations (TAMs), or mutations conferring multi-NRTI resistance were detected among HIV seroconverters.24
In the VOICE trial (NCT00705679), which assessed the effectiveness of tenofovir 1% vaginal gel and two different oral HIV prevention methods (tenofovir DF and emtricitabine/tenofovir DF [Truvada]), one participant in the Truvada group who underwent HIV-1 seroconversion 11 months after enrollment developed the M184V HIV resistance mutation.25
Select Clinical Trials
Tenofovir-based microbicides for rectal use
Rectal gel
Study Identifiers: MTN-017; NCT01687218
Sponsor: CONRAD
Phase: 2
Status: This study has been completed.
Study Purpose: The purpose of this study was to evaluate the safety and acceptability of reduced-glycerin tenofovir 1% gel applied rectally (either once daily or before and after sex [intermittently]) versus daily oral Truvada.
Study Population: Participants were sexually active male adults without HIV.5
Selected Study Results: Results published in Clinical Infectious Diseases (2017) showed that reduced-glycerin tenofovir gel applied rectally was safe. Product adherence was comparable for both the intermittent gel and the daily oral Truvada regimens, but was lower for the daily gel regimen. In terms of acceptability, participants liked using daily oral Truvada significantly more than either of the rectal gel regimens. When assessing ease of use and likelihood of future use for each regimen, there was no statistically significant difference between the intermittent gel and the daily oral Truvada regimens.26
Additional Published Material:
- AIDS Behav article, 2017: Preference of oral tenofovir disoproxil fumarate/emtricitabine versus rectal tenofovir reduced-glycerin 1% gel regimens for HIV prevention among cisgender men and transgender women who engage in receptive anal intercourse with men
- PLoS One article, 2017: High levels of adherence to a rectal microbicide gel and to oral Pre-Exposure Prophylaxis (PrEP) achieved in MTN-017 among men who have sex with men (MSM) and transgender women
Additional clinical trials of tenofovir 1% rectal gel have been completed, including:
- MTN-007 (NCT01232803): A Phase 1 study that evaluated the safety and acceptability of reduced glycerin tenofovir 1% gel for rectal use.27 Results are available from PLoS One (2013).
- CHARM-01 (NCT01575405) and CHARM-02 (NCT01575418): Phase 1 studies that evaluated the safety, acceptability, and pharmacokinetics of three gel formulations used rectally: a vaginal formulation, a reduced glycerin vaginal formulation, and a rectal-specific formulation.28,29 Results are available from PLoS One (2015) and AIDS Res Hum Retroviruses (2015).
Other rectal dosage forms
A tenofovir rectal douche or enema has been studied in four completed Phase 1 trials: DREAM-01 (NCT02750540), DREAM-02 (NCT04195776), DREAM-03 (NCT04016233), and ATN DREAM (NCT04686279).9,10,30,31 Results to DREAM-01 are published in J Infect Dis (2024) and Sex Transm Infect (2024). DREAM-03 results are available from CROI 2022. ATN DREAM results are available from ClinicalTrials.gov.
Rectal inserts containing tenofovir alafenamide/elvitegravir are also in development. A Phase 1 study (MTN-039; NCT04047420) was completed and results are available from J Infect Dis (2024) and CROI 2023.19 Another Phase 1 study (RITE Study; NCT06274398) is currently recruiting participants.12
Tenofovir-based vaginal microbicides
Vaginal gel
Study Identifiers: FACTS 001; NCT01386294
Sponsor: CONRAD
Phase: 3
Status: This study has been completed.
Study Purpose: The purpose of this study was to assess the safety and effectiveness of tenofovir 1% vaginal gel for preventing HIV infection in women.
Study Population: Participants were sexually active women aged 18-30 years in South Africa who did not have HIV.4,32
Selected Study Results: Results published in The Lancet Infectious Diseases (2018) showed that pericoital use of the tenofovir gel appeared safe but did not prevent HIV infection in young women. The HIV incidence rate was the same in both the tenofovir gel and placebo groups — 4.0 per 100 woman-years.32
Additional clinical trials evaluating tenofovir vaginal gel have also been completed. These include the Phase 2b CAPRISA 004 trial (NCT00441298) and the Phase 2b VOICE trial (MTN-003; NCT00705679).33,34 Results to CAPRISA 004 are published in Science (2010) and results to the VOICE trial are available from the N Engl J Med (2015).
Intravaginal rings
Study Identifiers: Protocol B17-144; NCT03762382
Sponsor: CONRAD
Phase: 2a
Status: This study has been completed.
Study Purpose: The primary purpose of this study was to evaluate the safety of a multipurpose prevention technology IVR delivering both tenofovir and levonorgestrel and a tenofovir only IVR, each compared to a placebo IVR, in women from Kenya. Secondarily, researchers assessed the pharmacokinetics and pharmacodynamics of each IVR, as well as IVR tolerability and acceptance.
Study Population: Participants were healthy, non-pregnant women without HIV, assessed to be at lower risk for acquiring HIV, in Western Kenya.8
Selected Study Results: Results published in Frontiers in Reproductive Health (2023) showed that both the tenofovir and levonorgestrel IVR and the tenofovir only IVR used continuously for 90 days were safe. No genital lesions were seen by visual inspection. Assessment of pharmacokinetic data and markers of protection indicate the potential efficacy of the multipurpose tenofovir/levonorgestrel IVR in preventing HIV, HSV-2, and unintended pregnancy.35
Additional Published Material:
- Sci Rep article, 2022: Genital microbiota of women using a 90 day tenofovir or tenofovir and levonorgestrel intravaginal ring in a placebo controlled randomized safety trial in Kenya
Additional Phase 1 studies of IVRs containing tenofovir have been conducted, including:
- MTN-038 (NCT03670355): A safety and pharmacokinetic study of a 90-day IVR containing tenofovir. This study has been completed, and results are published in J Int AIDS Soc (2024).36
- CONRAD A13-128 (NCT02235662): A one-month safety, pharmacokinetic, and pharmacodynamic study of the tenofovir/levonorgestrel IVR and a tenofovir-only IVR. This study has been completed, and results are available from PLoS One (2018).37
- CONRAD A15-138 (NCT03279120): A safety, pharmacokinetic, and pharmacodynamic study of a 90-day tenofovir/levonorgestrel IVR. This study has been completed. Results are published in PLoS One (2022).38
- NCT03255915: A pilot study evaluating the safety and pharmacokinetics of an IVR delivering both tenofovir DF and emtricitabine (TDF-FTC pod-IVR). This study is ongoing, but not recruiting participants.39
Other vaginal dosage forms
Other forms of tenofovir-based vaginal microbicides have been investigated in early-phase trials. These include a Phase 1 trial (NCT01694407) that evaluated vaginal tablets containing tenofovir and/or emtricitabine and a Phase 1 trial (FAME-04; NCT01989663) that assessed tenofovir vaginal gel and film formulations.6,7 Results to FAME-04 are available from J Int AIDS Soc (2018).
Vaginal inserts containing tenofovir alafenamide and elvitegravir are also under development. A Phase 1 study (NCT03762772) was completed, and results results are available from Front Cell Infect Microbiol (2023).18 Another Phase 1 study (MATRIX-001; NCT06087913) is currently recruiting participants.11
Adverse Events
MTN-017 (NCT01687218)
In this Phase 2 study, which compared rectally applied reduced glycerin tenofovir 1% gel with oral Truvada, the gel was reported to be safe, with the majority of adverse events (AEs) being mild or moderate in severity. Grade 2 or higher AE rates in the gel groups were similar to the rate in the oral Truvada group. Excluding rectal infections, the most common Grade 2 AE with the daily rectal gel and oral daily Truvada was diarrhea and headache, respectively.26
FACTS 001 (NCT01386294)
In this Phase 3 study, which compared tenofovir 1% vaginal gel with a placebo gel, a higher incidence of Grade 2 AEs was observed among participants in the tenofovir group than in the placebo group. The most common Grade 2 or higher product-related AEs were hypophosphataemia, genital symptoms, or elevated transaminases. No product-related serious AEs occurred during the trial. There were no notable differences in product-related AEs, Grade 3 AEs, or Grade 4 AEs between treatment groups.32
Protocol B17-144 (NCT03762382)
In this Phase 2a trial evaluating the continuous use of a tenofovir/levonorgestrel IVR and a tenofovir-only IVR against a placebo IVR for up to 90 days, the most common AEs that occurred were bacterial vaginosis, headache, and respiratory tract infections. The only product-related AEs that occurred were Grade 1 or 2 changes in menstrual bleeding patterns, which were reported in 6 participants using the tenofovir/levonorgestrel IVR and 1 participant using the tenofovir-only IVR. There were no genital lesions observed by visual inspection.35
Drug Interactions
A Phase 1 pharmacokinetic study evaluated the effect of oral contraceptive (levonorgestrel/ethinyl estradiol) or depot medroxyprogesterone acetate use in women using tenofovir vaginal gel. Results demonstrated that contraceptive hormone use caused a significant decrease in concentrations of tenofovir in cervicovaginal aspirate. However, investigators determined that tenofovir concentrations still remained at sufficiently high levels to maintain mucosal anti-HIV efficacy.40,41
References
- National Center for Biotechnology Information. PubChem compound summary for CID 464205, tenofovir. Accessed September 16, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). NIAID ChemDB, HIV Drugs in Development. Accessed September 16, 2024
- Abdool Karim SS, Baxter C, Abdool Karim Q. Advancing HIV prevention using tenofovir-based pre-exposure prophylaxis. Antivir Ther. 2022;27(2):13596535211067588. doi:10.1177/13596535211067589. Accessed September 16, 2024
- CONRAD. A Phase III, multi-centre, randomized controlled trial to assess the safety and effectiveness of the vaginal microbicide 1% tenofovir gel in the prevention of human immunodeficiency virus type 1 infection in women, and to examine effects of the microbicide on the incidence of herpes simplex virus type 2 infection. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 28, 2011. NLM Identifier: NCT01386294. Accessed September 16, 2024
- CONRAD. A Phase 2 randomized sequence open label expanded safety and acceptability study of oral emtricitabine/tenofovir disoproxil fumarate tablet and rectally-applied tenofovir reduced-glycerin 1% gel. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on August 27, 2012. NLM Identifier: NCT01687218. Accessed September 16, 2024
- CONRAD. A Phase I clinical trial assessing the safety, pharmacokinetics, pharmacodynamics, and disintegration time of vaginal tablets containing tenofovir and/or emtricitabine. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 17, 2012. NLM Identifier: NCT01694407. Accessed September 16, 2024
- CONRAD. A Phase I trial to assess the safety of tenofovir gel and film formulations: FAME 04. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 5, 2013. NLM Identifier: NCT01989663. Accessed September 16, 2024
- CONRAD. Phase IIa, 90-day safety, adherence, and acceptability study of intravaginal rings releasing tenofovir with and without levonorgestrel among women in Western Kenya. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 16, 2018. NLM Identifier: NCT03762382. Accessed September 16, 2024
- Johns Hopkins University. A Phase 1 open label study evaluating the distribution of a tenofovir douche in combination with tap water douching and simulated receptive anal intercourse (DREAM-02). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on December 10, 2019. NLM Identifier: NCT04195776. Accessed September 16, 2024
- Johns Hopkins University. A Phase I, open-label multiple dose safety, pharmacokinetic, pharmacodynamic, and acceptability study of tenofovir rectal douche. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 9, 2019. NLM Identifier: NCT04016233. Accessed September 16, 2024
- Eastern Virginia Medical School. A Phase I randomized, placebo-controlled, double-blind study to assess safety, pharmacokinetics, and modeled pharmacodynamics of a vaginal insert containing tenofovir alafenamide and elvitegravir. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on September 7, 2023. NLM Identifier: NCT06087913. Accessed September 16, 2024
- Eastern Virginia Medical School. A double-blind, placebo-controlled, randomized Phase 1 study to evaluate the safety and pharmacokinetics of rectally administered tenofovir alafenamide/elvitegravir inserts. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 31, 2024. NLM Identifier: NCT06274398. Accessed September 16, 2024
- Shattock RJ, Rosenberg Z. Microbicides: topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2(2):a007385. Accessed September 16, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). Microbicides to block transmission of HIV. Accessed September 16, 2024
- Gengiah TN, Baxter C, Mansoor LE, Kharsany AB, Abdool Karim SS. A drug evaluation of 1% tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection. Expert Opin Investig Drugs. 2012;21(5):695-715. doi:10.1517/13543784.2012.667072. Accessed September 16, 2024
- Balzarini J, Van Damme L. Intravaginal and intrarectal microbicides to prevent HIV infection. CMAJ. 2005;172(4):461-464. doi:10.1503/cmaj.1041462. Accessed September 16, 2024
- Verma NA, Lee AC, Herold BC, Keller MJ. Topical prophylaxis for HIV prevention in women: becoming a reality. Curr HIV/AIDS Rep. 2011;8(2):104-113. doi:10.1007/s11904-011-0075-7. Accessed September 16, 2024
- CONRAD. A Phase I study to assess safety, pharmacokinetics, and pharmacodynamics of a vaginal insert containing tenofovir alafenamide and elvitegravir. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 16, 2018. NLM Identifier: NCT03762772. Accessed September 16, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). A Phase 1 open label safety and pharmacokinetic study of rectal administration of a tenofovir alafenamide/elvitegravir insert at two dose levels. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on August 5, 2019. NLM Identifier: NCT04047420. Accessed September 16, 2024
- Rees H, Delany-Moretlwe SA, Lombard C, et al. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. Abstract presented at: 22nd Conference on Retroviruses and Opportunistic Infections (CROI); February 23-26, 2015; Seattle, Washington. Abstract 26LB. Accessed September 16, 2024
- Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86(2):718-725. doi:10.1128/JVI.05842-11. Accessed September 16, 2024
- Yang KH, Hendrix C, Bumpus N, et al. A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate. PLoS ONE. 2014;9(10):e106196. doi:10.1371/journal.pone.0106196. Accessed September 16, 2024
- Klatt NR, Cheu R, Birse K, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356(6341):938-945. doi:10.1126/science.aai9383. Accessed September 16, 2024
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168-1174. doi:10.1126/science.1193748. Accessed September 16, 2024
- Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509-518. Accessed September 16, 2024
- Cranston RD, Lama JR, Richardson BA, et al. MTN-017: a rectal Phase 2 extended safety and acceptability study of tenofovir reduced-glycerin 1% gel. Clin Infect Dis. 2017;64(5):614-620. doi:10.1093/cid/ciw832. Accessed September 16, 2024
- CONRAD. A Phase 1 randomized, double-blinded, placebo-controlled rectal safety and acceptability study of tenofovir 1% gel. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 1, 2010. NLM Identifier: NCT01232803. Accessed September 16, 2024
- Ian McGowan. A randomized, double blind Phase 1 safety, acceptability, and pharmacokinetic study comparing three formulations of tenofovir 1% gel administered rectally to HIV-1 seronegative adults. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on February 29, 2012. NLM Identifier: NCT01575405. Accessed September 16, 2024
- Ian McGowan. An exploratory, double-blinded, randomized, pharmacokinetic and safety study of three rectally-applied tenofovir 1% microbicide gel formulations. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on March 1, 2012. NLM Identifier: NCT01575418. Accessed September 16, 2024
- Johns Hopkins University. DREAM-01: optimization of a tenofovir enema for HIV prevention. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on April 11, 2016. NLM Identifier: NCT02750540. Accessed September 16, 2024
- University of Pennsylvania. Safety, PK/PD, acceptability, and desirability of a novel HIV prevention douche among adolescent men (DREAM). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on December 22, 2020. NLM Identifier: NCT04686279. Accessed September 16, 2024
- Delany-Moretlwe S, Lombard C, Baron D, et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2018;18(11):1241-1250. doi:10.1016/S1473-3099(18)30428-6. Accessed September 16, 2024
- Centre for the AIDS Programme of Research in South Africa. Phase IIb trial to assess the safety and effectiveness of the vaginal microbicide 1% tenofovir gel for the prevention of HIV infection in women in South Africa. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on February 27, 2007. NLM Identifier: NCT00441298. Accessed September 16, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). Phase 2B safety and effectiveness study of tenofovir 1% gel, tenofovir disproxil fumarate tablet and emtricitabine/tenofovir disoproxil fumarate tablet for the prevention of HIV infection in women. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 24, 2008. NLM Identifier: NCT00705679. Accessed September 16, 2024
- Mugo NR, Mudhune V, Heffron R, et al. Randomized controlled phase IIa clinical trial of safety, pharmacokinetics and pharmacodynamics of tenofovir and tenofovir plus levonorgestrel releasing intravaginal rings used by women in Kenya. Front Reprod Health. 2023;5:1118030. doi:10.3389/frph.2023.1118030. Accessed September 16, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). A Phase 1, randomized pharmacokinetic and safety study of a 90 Day intravaginal ring containing tenofovir. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on September 12, 2018. NLM Identifier: NCT03670355. Accessed September 16, 2024
- CONRAD. Phase I one-month safety, pharmacokinetic, pharmacodynamic, and acceptability study of intravaginal rings releasing tenofovir and levonorgestrel or tenofovir alone. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 14, 2014. NLM Identifier: NCT02235662. Accessed September 16, 2024
- CONRAD. Phase I, 90-Day safety, pharmacokinetic, and pharmacodynamic study of intravaginal rings releasing tenofovir and levonorgestrel. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on August 23, 2017. NLM Identifier: NCT03279120. Accessed September 16, 2024
- Oak Crest Institute of Science. Randomized order, controlled, double blind, crossover early Phase 1 pilot study to assess safety and pharmacokinetics of a tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) releasing IVR over 28 days compared to placebo. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 26, 2017. NLM Identifier: NCT03255915. Accessed September 16, 2024
- CONRAD. Assessing the effect of contraception and the menstrual cycle on pharmacokinetics, pharmacodynamics, and vaginal safety in tenofovir vaginal gel users. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on August 9, 2011. NLM Identifier: NCT01421368. Accessed September 16, 2024
- Thurman AR, Schwartz JL, Brache V, et al. Effect of hormonal contraception on pharmacokinetics of vaginal tenofovir in healthy women: increased tenofovir diphosphate in injectable depot medroxyprogesterone acetate users. J Acquir Immune Defic Syndr. 2019;80(1):79-88. doi:10.1097/QAI.0000000000001864. Accessed September 16, 2024
Last Reviewed: September 16, 2024