Drug information
Audio
| drug-audio-en-420.mp3 |
Pronounce:
Brand Name
Isentress, Isentress HD
Other Names
RAL, raltegravir potassium
Drug Class
Integrase Strand Transfer Inhibitor (INSTIs)
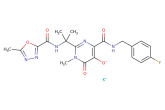
raltegravir potassium
Molecular Weight: 482.511
(The drug image[s] shown above are of brand products only. There may be other available products not shown.)
FDA label informationFDA label information
Several FDA-approved drug labels may be available for raltegravir. Clinicalinfo provides the following link to the DailyMed drug label solely as an example of the labels available for raltegravir. Inclusion or absence of a drug label link on the Clinicalinfo site does not imply endorsement or lack thereof by Clinicalinfo. Search DailyMed or Drugs@FDA to access more information on raltegravir, including additional drug labels and any generic equivalents.
Tablet (chewable), tablet (film coated), granule for oral suspension