Drug information
| drug-audio-en-Elsulfavirine.mp3 |
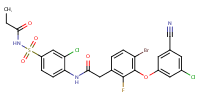
C24 H17 Br Cl2 F N3 O5 S
N-(4-((2-(4-Bromo-3-(3-chloro-5-cyano-phenoxy)-2-fluoro-phenyl)acetyl)amino)-3-chloro-phenyl)sulfonylpropanamide
Elsulfavirine has been studied in a Phase 2/3 trial for HIV treatment. In June 2017, elsulfavirine received marketing approval in Russia for the treatment of HIV.
elsulfavirine
Molecular Weight: 629.2823
(Compound details obtained from PubChem,1 NIAID Therapeutics Database,2 Viriom press release,3 and ClinicalTrials.gov4)
Pharmacology
Mechanism of Action
Non-nucleoside reverse transcriptase inhibitor (NNRTI). Elsulfavirine (brand name: Elpida) is a prodrug of VM-1500A, a selective NNRTI with potent activity against HIV-1.5,6 NNRTIs generally function by inhibiting HIV-1 reverse transcriptase and preventing the conversion of viral RNA to DNA.7,8 Elsulfavirine has been developed as a once-daily oral treatment for HIV and has been studied in a Phase 2/3 trial. In June 2017, elsulfavirine was granted marketing approval in Russia for treating HIV.3,4,9
Investigators are also examining once-weekly oral dosing of elsulfavirine for HIV treatment. Fixed-dose combinations (FDCs) containing elsulfavirine and a long-acting injectable formulation of VM-1500A are also in development for HIV treatment. In addition, the long-acting injectable formulation of VM-1500A is currently in Phase 2/3 development for HIV treatment, pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP).10,11
Half-life (T½)
Following single oral doses of elsulfavirine in healthy participants without HIV, the mean half-life of elsulfavirine was 1.7 hours (20-mg dose) and 2.6 hours (40-mg dose). The half-life of VM-1500A, the active metabolite, was 8.9 days (20-mg dose) and 8.8 days (40-mg dose).12
In a study of treatment-naive participants with HIV, the half-life of elsulfavirine following a single oral dose was 1.9 hours (20-mg dose) and 2.1 hours (40-mg dose). After 7 days of once-daily administration, the half-life of elsulfavirine was 2.4 hours (40-mg dose). The half-life of the VM-1500A metabolite after 7 days was 7.4 days (20-mg dose) and 5.4 days (40-mg dose).12
Select Clinical Trials
Study Identifier: NCT02485509
Sponsor: Viriom
Phase: 1b/2a
Status: This study has been completed.
Study Purpose: The purpose of this two-part study was to 1) assess the safety and pharmacokinetics of elsulfavirine in healthy participants without HIV, and 2) evaluate the pharmacokinetics, safety, and antiviral activity of elsulfavirine in participants with HIV.
Study Population:
- Part 1: Participants were healthy adults without HIV.
- Part 2: Participants were treatment-naive adults with HIV.13
Selected Study Results: Results presented at CROI 2014 showed that elsulfavirine at both the 20 mg and 40 mg doses was effective with minimal side effects. A 1.8 log reduction in HIV-1 RNA was noted across both dosage groups. Participants who received treatment with elsulfavirine 20 mg had an average CD4 count increase from 551 cells/mm3 to 592 cells/mm3 and participants receiving elsulfavirine 40 mg had an increase in CD4 count from 471 cells/mm3 to 525 cells/mm3.14
Additional Published Material:
Study Identifier: NCT02489461
Sponsor: Viriom
Phase: 2/3
Status: This study has been completed.
Study Purpose: The purpose of this study was to 1) select an optimal dose of elsulfavirine and 2) compare the safety and effectiveness of the optimal elsulfavirine dose to efavirenz (EFV).
Study Population:
- Participants were treatment-naive adults with clinically stable (World Health Organization [WHO] clinical stage 1 or 2) HIV infection.
- Participants had HIV RNA ≥5,000 copies/mL and CD4 counts >200 cells/mm3 at screening.4,5
Selected Study Results: Results presented at CROI 2017 showed that elsulfavirine was safe and effective. The CD4 counts of participants increased at Week 48 by 179 cells/mm3 in participants receiving elsulfavirine and by 182 cells/mm3 in participants receiving efavirenz. Additionally, at Week 48, 81% of participants receiving elsulfavirine and 73.7% of participants receiving efavirenz had HIV RNA <400 copies/mL.5
Additional Published Material:
- EAC, 2015: Safety and antiviral effect of Elpida (VM-1500), a novel NNRTI (+Truvada) in treatment-naive HIV-1 infected patients
Additional clinical trials of elsulfavirine have also been conducted, including:
- NCT05165550: A Phase 1 trial evaluating the safety, tolerability, and pharmacokinetics of single oral ascending doses of elsulfavirine in healthy adults without HIV. This study has been completed.15
- NCT03730311: A Phase 1b trial investigating the safety, tolerability and pharmacokinetics of once-weekly oral elsulfavirine in healthy adults without HIV. The status of this study is unknown.16
Adverse Events
NCT02485509:
In Part 2 of this Phase 1b/2a trial, eight total participants were randomized to the 20-mg group (n=7 elsulfavirine; n=1 placebo). Three of the eight participants experienced mild dry mouth and polyuria and one participant had mild headache. No adverse events (AEs) were reported among participants receiving elsulfavirine 40 mg.13,14
NCT02489461:
In Part 1 of this Phase 2/3 trial, a safety analysis was performed on data from 30 participants receiving elsulfavirine 20 mg, 29 participants receiving elsulfavirine 40 mg, and 28 participants receiving EFV. Overall, drug-related AEs occurred in 3.3% of participants on elsulfavirine 20 mg, 13.8% of participants on elsulfavirine 40 mg, and 46.4% of participants on EFV. Three participants experienced a serious adverse event (SAE), but none of the events were likely related to study treatment. Neurologic and psychiatric AEs that were definitely associated with treatment during the study occurred in a greater proportion of participants receiving EFV than in participants receiving elsulfavirine. A greater variety of neurologic and psychiatric events commonly occurred in the EFV group compared to either of the elsulfavirine groups. Commonly occurring neurologic events in the elsulfavirine groups were headache (seen in both the 20- and 40-mg groups) along with unusual dreams, dizziness, sleep disturbance, and drowsiness (seen in the 40-mg group). Common psychiatric events in the elsulfavirine groups included sleep disturbances (seen in both the 20-mg and 40-mg groups) plus depression and nightmares (seen in the 20-mg group).4,6
During Stage 2 of the trial, the safety of elsulfavirine was assessed among 60 elsulfavirine participants and 58 EFV participants. Drug-related AEs occurred in 36.7% of elsulfavirine participants versus 77.6% of EFV participants. Three participants receiving elsulfavirine experienced three SAEs, but none of the events were drug-related; seven participants receiving EFV experienced eight SAEs, three of which were not drug-related and five of which were probably drug-related. Study discontinuations due to an AE occurred in one participant receiving elsulfavirine versus seven participants receiving EFV. CNS and skin AEs occurring in more than 5% of participants were reported in a greater proportion of EFV participants (62.1%) than elsulfavirine participants (31.7%). The most common of these AEs were headache (15.0% elsulfavirine; 24.1% EFV), dizziness (6.7% elsulfavirine; 27.6% EFV), and sleep disorders (5% elsulfavirine; 20.7% EFV). Abnormal dreams (17.2%), skin rash (17.2%), and pruritus (5.2%) occurred only in EFV-treated participants.5
Drug Interactions
Drug-drug interactions associated with elsulfavirine are currently unknown.
References
- National Center for Biotechnology Information. PubChem compound summary for CID, 11527519, elsulfavirine. Accessed August 3, 2023
- National Institute of Allergy and Infectious Diseases (NIAID). NIAID ChemDB, HIV Drugs in Development. Accessed August 3, 2023
- Viriom. Press release, dated July 25, 2017. Viriom obtains first market approval of elsulfavirine (Elpida®) for treatment of HIV-1 infection in Russia. Accessed August 3, 2023
- Viriom. International, multicenter, randomized, partially blind clinical study to evaluate efficacy, safety and selection of the optimal dose for VM-1500 in comparison to efavirenz in combination with two NRTIs in treatment-naive, HIV-1 infected patients. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 18, 2015. NLM Identifier: NCT02489461. Accessed August 3, 2023
- Murphy RL, Kravchenko AV, Orlova-Morozova EA, et al. Elsulfavirine as compared to efavirenz in combination with TDF/FTC: 48-week study. Poster presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 13-16, 2017; Seattle, Washington. Poster 452LB. Accessed August 3, 2023
- Kravchenko AV, Orlova-Morozova EA, Nagimova FI, et al. Safety and antiviral effect of Elpida (VM-1500), a novel NNRTI (+Truvada) in treatment-naive HIV-1 infected patients. Poster presented at: European AIDS Conference (EAC); October 21-24, 2015; Barcelona, Spain. Poster PE7/4. Accessed August 3, 2023
- Sluis-Cremer N, Tachedjian G. Mechanisms of inhibition of HIV replication by nonnucleoside reverse transcriptase inhibitors. Virus Res. 2008;134(1-2):147-156. doi:10.1016/j.virusres.2008.01.002 Accessed August 3, 2023
- Schauer G, Leuba S, Sluis-Cremer N. Biophysical insights into the inhibitory mechanism of non-nucleoside HIV-1 reverse transcriptase inhibitors. Biomolecules. 2013;3(4):889-904. doi:10.3390/biom3040889. Accessed August 3, 2023
- Viriom, Inc. website. Pipeline. Accessed August 3, 2023
- Viriom. Multicenter, open-label, randomized, active control study to evaluate efficacy and safety of switching to VM-1500A-LAI + 2NRTIs from the 1st line standard of care therapy. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 10, 2022. NLM Identifier: NCT05204394. Accessed August 3, 2023
- Viriom. An open-label study to evaluate safety, tolerability and pharmacokinetics of VM-1500A-LAI after single and multiple ascending dose administration to healthy volunteers. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on October 11, 2018. NLM Identifier: NCT03706911. Accessed August 3, 2023
- Ratanasuwan W, Werarak P, Koryakova A, Berzins B, Bichko V, Murphy R. Pharmacokinetics of VM-1500 20 mg and 40 mg in healthy and HIV-infected patients. International AIDS Conference (AIDS); July 20-25, 2014; Melbourne, Australia. Levin: Conference reports for National AIDS Treatment Advocacy Project (NATAP); 2014. Accessed August 3, 2023
- Viriom. Phase Ib/IIa, single-centre, placebo-controlled randomized study of safety and pharmacokinetics in healthy volunteers and safety, tolerability and antiviral activity of VM-1500 in patients with human immunodeficiency virus-1 infection. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 18, 2015. NLM Identifier: NCT02485509. Accessed August 3, 2023
- Ratanasuwan W, Werarak P, Murphy RL, Bichko V. A randomized, placebo-controlled, double-blind study of VM-1500 in HIV-naive patients. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); March 3-6, 2014; Boston, Massachusetts. Abstract 544LB. Accessed August 3, 2023
- Viriom. A Phase 1, double-blind, placebo-controlled study of single ascending doses of elsulfavirine to evaluate the safety, tolerability, and pharmacokinetics of elsulfavirine and its active metabolite VM-1500A in healthy subjects. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on December 7, 2021. NLM Identifier: NCT05165550. Accessed August 3, 2023
- Viriom. Phase Ib, single-centre, placebo-controlled randomised study of safety, tolerability and pharmacokinetics of Elpida in healthy HIV-uninfected volunteers. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 1, 2018. NLM Identifier: NCT03730311. Accessed August 3, 2023
Last Reviewed: August 3, 2023