Drug information
| drug-audio-en-Panobinostat.mp3 |
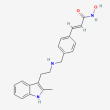
C21 H23 N3 O2
2-Propenamide, N-hydroxy-3-(4-(((2-(2-methyl-1H-indol-3-yl)ethyl)amino)methyl)phenyl)-, (2E)-
Panobinostat is in Phase 1/2 development as a latency-reversing agent for HIV treatment.
panobinostat
Molecular Weight: 349.4
(Compound details obtained from PubChem,1 Treatment Action Group website,2 Farydak Summary of Product Characteristics,3 and Journal of Biomedicine and Biotechnology article4)
Pharmacology
Mechanism of Action
Latency-reversing agent, specifically a histone deacetylase inhibitor (HDACi).2 Panobinostat, a cinnamic hydroxamic acid analogue, is a pan-HDACi that targets many Class I, II, and IV histone deacetylases (HDACs), including the Class I HDAC-1, -2, and -3 enzymes, which are important in disruption of HIV latency.5-7 Panobinostat was previously FDA approved under the brand name Farydak for the treatment of multiple myeloma and is no longer marketed in the United States; it is still available in other parts of the world.3,8 As an HIV therapeutic, panobinostat is currently being investigated as an agent for reactivating latent HIV expression.2,9 In HIV-1 latency, HDACs are recruited to the proviral 5' long terminal repeat (LTR), where they catalyze deacetylation of lysine residues on histones. This results in chromatin condensing on nucleosome 1 (nuc-1), which prevents HIV transcription. Inhibition of HDAC activity promotes histone acetylation (hyperacetylation) of lysine residues by histone acetyltransferases (HATs), leading to chromatin relaxation and transcriptional activation.7,10 Some research suggests that the activity of HDACs in inducing HIV transcription may not be caused by direct effects on histone acetylation, but may be caused by effects on other nonhistone proteins.6,11,12
Half-life (T½)
In participants with advanced cancer, the estimated terminal elimination half-life of panobinostat was 37 hours.3
Metabolism/Elimination
Panobinostat is extensively metabolized via reduction, hydrolysis, oxidation, and glucuronidation. Approximately 40% of the total dose of panobinostat is eliminated by oxidative metabolism, primarily via CYP3A4. Minor contributions to the metabolism of panobinostat are mediated by CYP2D6 and CYP2C19 enzymes.3
After a single oral dose of radiolabeled panobinostat in participants with advanced cancer, 29% to 51% of the administered dose was excreted in urine (with less than 2.5% as unchanged drug), and 44% to 77% was excreted in feces (with less than 3.5% as unchanged drug).3
Select Clinical Trials
Study Identifiers: CLEAR; NCT01680094
Sponsor: University of Aarhus
Phase: 1/2
Status: This study has been completed.
Study Purpose: The purpose of this open-label study was to evaluate the safety and efficacy of panobinostat in reactivating HIV transcription in latently infected CD4 cells.
Study Population:
- Participants were adults with HIV who had received continuous ART for at least 2 years prior to enrollment.
- Participants were virologically suppressed with HIV RNA <50 copies/mL for at least 2 years and had CD4 counts >500 cells/mm3 at last measurement.6,13
Selected Study Results: Results published in Lancet HIV (2014) showed that panobinostat administered to virologically suppressed participants on ART was effective in reactivating latent HIV. However, panobinostat did not reduce the size of the latent HIV reservoir.14
Additional Published Material:
- IAS Towards an HIV Cure Symposium, 2013: Cyclic panobinostat (LBH589) dosing in HIV-1 patients: findings from the CLEAR trial
- CROI, 2015: Panobinostat broadly activates latent HIV-1 proviruses in patients
Study Identifiers: ACTIVATE; NCT02471430
Sponsor: Massachusetts General Hospital
Phase: 1/2
Status: This study has been completed.
Study Purpose: The purpose of this open-label study was to evaluate whether a combination regimen of panobinostat and the immunomodulator peginterferon alfa-2a could reduce the latent HIV reservoir.
Study Population:
- Participants were adults with HIV who had been receiving continuous ART for at least 24 months prior to screening and who had been receiving the same ART regimen for at least 12 weeks prior to screening.
- Participants were virologically suppressed on ART, with HIV RNA <50 copies/mL, for at least 24 months prior to screening and had CD4 counts ≥400 cells/mm3.15
Selected Study Results: Results published in Cell (2024) and presented at CROI 2022 indicated that panobinostat and interferon alfa-2a reactivated latent HIV and induced immune activation in participants with virological suppression on ART. The combination regimen produced no major change in total HIV-1 proviral DNA, but a trend for a decrease of intact proviruses was seen.16,17
Study Identifiers: ORBIT; NCT06240520
Sponsor: Erasmus Medical Center
Phase: 1/2
Status: See the ClinicalTrials.gov record for this study’s status.
Study Purpose: The purpose of this open-label study is to evaluate the ability of pyrimethamine, lenalidomide, and panobinostat combination therapy to reactivate latent HIV.
Study Population:
- Participants are adults with subtype B HIV who have been receiving uninterrupted ART for at least 2 consecutive years.
- Participants have a WHO performance status of 0 or 1 and no clinical signs of cellular immunodeficiency or AIDS.
- Participants have HIV RNA <50 copies/mL and CD4 counts ≥200 cells/mm3.
- Participants had HIV RNA ≥1,000 copies/mL prior to initiating ART.9
Adverse Events
CLEAR (NCT01680094)
In the Phase 1/2 CLEAR trial, 45 adverse events (AEs) were reported, including 16 related to panobinostat. The panobinostat-related AEs were all Grade 1 in severity. The most frequently occurring panobinostat-related AE was fatigue. Declines in neutrophil and thrombocyte counts noted during the study were reversible and within normal limits, respectively. Panobinostat treatment did not alter CD4 counts.6,14 In a substudy analysis, researchers evaluated cerebrospinal fluid (CSF) inflammation and neurodegeneration biomarkers and found that panobinostat did not result in central nervous system (CNS) AEs.18,19
Panobinostat caused significant immunomodulatory changes, but these changes did not appear harmful and did not persist beyond 4 weeks after the end of dosing. Alterations in gene expression that had occurred during panobinostat dosing returned to normal by 24 weeks postdosing.20 Furthermore, analysis of panobinostat’s effect on HIV-specific CD8 cells found no evidence that panobinostat decreased levels or responses of HIV-1 specific effector memory (EM) CD8 cells.21
ACTIVATE (NCT02471430)
In the Phase 1/2 ACTIVATE trial, participants on ART were randomized to receive either panobinostat alone, panobinostat plus peginterferon alfa-2a, or peginterferon alfa-2a alone. Fifteen AEs occurred during the study, most of which were Grade 1 or 2. The most common AEs associated with peginterferon alfa-2a were body aches and fatigue, and the most common AEs associated with panobinostat were nausea and mild diarrhea. One case of self-limiting Grade 3 neutropenia occurred in a participant receiving panobinostat plus peginterferon alfa-2a. There were no serious AEs reported.15,16
Drug Interactions
Panobinostat undergoes non-CYP mediated metabolism (reduction, hydrolysis, oxidation, and glucuronidation), as well as CYP mediated metabolism. Approximately 40% of a panobinostat dose is metabolized by CYP3A4, with minor contributions from CYP2D6 and CYP2C19. Panobinostat pharmacokinetics may therefore be affected by CYP3A4 inhibitors and inducers. Panobinostat is also a P-gp substrate.3
Drug-drug interactions between panobinostat and concomitant medications have been previously described. These include interactions between panobinostat and strong CYP3A and/or P-gp inhibitors, including the HIV PIs ritonavir and saquinavir; strong CYP3A inducers; sensitive CYP2D6 substrates or CYP2D6 substrates that have a narrow therapeutic index; and drugs that are known to prolong the QT interval.3
References
- National Center for Biotechnology Information: PubChem compound summary for CID 6918837, panobinostat. Accessed May 9, 2024
- Treatment Action Group. Research toward a cure trials. June 2020. Accessed May 9, 2024
- pharmaand GmbH. Farydak 10mg hard capsules summary of product characteristics (SmPC), September 30, 2023. Electronic Medicines Compendium (EMC). Accessed May 9, 2024
- Masetti R, Serravalle S, Biagi C, Pession A. The role of HDACs inhibitors in childhood and adolescence acute leukemias. J Biomed Biotechnol. Published online Article ID 148046 2011. doi:10.1155/2011/148046. Accessed May 9, 2024
- Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 2013;21(6):277-285. doi:10.1016/j.tim.2013.02.005. Accessed May 9, 2024
- Tolstrup M. Cyclic Panobinostat (LBH589) dosing in HIV-1 patients: findings from the CLEAR trial. Slides presented at: International AIDS Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention; June 30–July 3, 2013; Kuala Lumpur, Malaysia. Accessed May 9, 2024
- Matalon S, Rasmussen TA, Dinarello CA. Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med. 2011;17(5-6):466-472. doi:10.2119/molmed.2011.00076. Accessed May 9, 2024
- Secura Bio, Inc.: Press Release, dated November 30, 2021. Secura Bio announces U.S. withdrawal of FARYDAK ® (panobinostat) NDA. Accessed May 9, 2024
- Erasmus Medical Center. Optimizing reversal of HIV latency with combination therapy (pyrimethamine, lenalidomide, panobinostat). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 26, 2024. NLM Identifier: NCT06240520. Accessed May 9, 2024
- Rasmussen TA, Tolstrup M, Winckelmann A, Østergaard L, Søgaard OS. Eliminating the latent HIV reservoir by reactivation strategies. Hum Vaccines Immunother. 2013;9(4):790–799. Accessed May 9, 2024
- Elliott JH, Wightman F, Solomon A, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10(11).doi:10.1371/journal.ppat.1004473. Accessed May 9, 2024
- Jamaluddin MS, Hu PW, Jan Y, Siwak EB, Rice AP. Short communication: the broad-spectrum histone deacetylase inhibitors vorinostat and panobinostat activate latent HIV in CD4(+) T cells in part through phosphorylation of the T-loop of the CDK9 subunit of P-TEFb. AIDS Res Hum Retroviruses. 2016;32(2):169-173. doi:10.1089/aid.2015.0347. Accessed May 9, 2024
- University of Aarhus. The safety and efficacy of the histone deacetylase inhibitor panobinostat for purging HIV-1 from the latent reservoir (CLEAR) study. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on September 3, 2012. NLM Identifier: NCT01680094. Accessed May 9, 2024
- Rasmussen TA, Tolstrup M, Brinkmann CR, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a Phase 1/2, single group, clinical trial. Lancet HIV. 2014;1(1):e13-21. doi:10.1016/S2352-3018(14)70014-1. Accessed May 9, 2024
- Massachusetts General Hospital. A Phase I-II pilot study to assess the safety and efficacy of combined administration with pegylated interferon-alpha2a and the histone deacetylase inhibitor (HDACi) panobinostat for reducing the residual reservoir of HIV-1 infected cells in cART-treated HIV-1 positive individuals. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 11, 2015. NLM Identifier: NCT02471430. Accessed May 9, 2024
- Armani-Tourret M, Gao C, Hartana CA, et al. Selection of epigenetically privileged HIV-1 proviruses during treatment with panobinostat and interferon-α2a. Cell. 2024;187(5):1238-1254.e14. doi:10.1016/j.cell.2024.01.037. Accessed May 9, 2024
- Armani-Tourret M, Hartana CA, Rassadkina Y, et al. HIV-1 viral reservoir disruption with panobinostat and IFN-⍺. Poster presented at: Conference on Retroviruses and Opportunistic Infections (CROI)); February 12-16, 2022; Virtual. Poster 357. Accessed May 9, 2024
- Rasmussen TA, Tolstrup M, Møller HJ, et al. Activation of latent human immunodeficiency virus by the histone deacetylase inhibitor panobinostat: a pilot study to assess effects on the central nervous system. Open Forum Infect Dis. 2015;2(1). doi:10.1093/ofid/ofv037. Accessed May 9, 2024
- Rasmussen TA, Søgaard OS, Møller HJ, et al. HIV reactivation by the histone deacetylase inhibitor panobinostat: effects on CNS. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); March 3-6, 2014; Boston, MA. Abstract 482. Accessed May 9, 2024
- Tolstrup M, Brinkmann CR, Rasmussen TA, Olesen R, Kj AS. Panobinostat dosing has broad but transient immunomodulatory effects in HIV-patients. Poster presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 23-26, 2015; Seattle, WA. Poster 405. Accessed May 9, 2024
- Olesen R, Rasmussen TA, Lichterfeld M, et al. In vivo effects of panobinostat and romidepsin on HIV-1-specific CD8 T cell immunity. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 23-26, 2015; Seattle, WA. Abstract 369. Accessed May 9, 2024
Last Reviewed: May 9, 2024