Drug information
| drug-audio-en-Islatravir.mp3 |
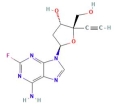
C12 H12 F N5 O3
4'-Ethynyl-2-fluoro-2'-deoxyadenosine
Islatravir is in Phase 3 development for HIV treatment. It is being developed as part of a fixed-dose combination containing doravirine and islatravir (DOR/ISL) and as a stand-alone agent. Islatravir is also being studied for HIV prevention; however, the development of once-monthly oral islatravir for HIV prevention is being discontinued.
islatravir
Molecular Weight: 293.25
(Compound details obtained from PubChem,1 NIAID Therapeutics Database,2 Treatment Action Group Pipeline Report 2023,3,4 and Merck press release5)
Pharmacology
Mechanism of Action
Nucleoside reverse transcriptase translocation inhibitor (NRTTI). Islatravir (ISL), a deoxyadenosine analog, belongs to a new class of ARV drugs called NRTTIs. It has potent activity against HIV-1 and is also active against HIV-2 and multidrug-resistant HIV strains.6,7
Intracellularly, ISL is converted to its active triphosphate form (ISL-TP). ISL-TP inhibits HIV reverse transcriptase (RT) through multiple modes of action. Primarily, ISL-TP functions as an immediate chain terminator—after ISL is incorporated into viral DNA, it blocks RT translocation and prevents nucleotide attachment onto the viral DNA chain. In instances where RT translocation does occur and additional nucleotides are allowed to incorporate onto the viral DNA chain, ISL-TP can act as a delayed chain terminator by causing structural changes to the viral chain. Furthermore, ISL can be misincorporated by RT, resulting in mismatched primers that cannot be extended or excised.6-9
Half-life (T½)
Following single-dose oral administration of ISL in adults without HIV, the apparent plasma half-life of ISL ranged from 49 to 61 hours.10,11 ISL-TP, the active form of ISL, has an intracellular half-life of 177 to 209 hours.11
Metabolism/Elimination
ISL undergoes adenosine deaminase-mediated metabolism and renal excretion; hepatic metabolism does not appear to have a significant role in ISL elimination.12 Following a single oral dose of radiolabeled ISL administered to healthy male participants, unchanged ISL was the predominant compound in plasma and 4′-ethynyl-2-fluorodeoxyinosine (M4) was the major metabolite in plasma. Ninety-one percent of the total radioactive dose was recovered in urine (with the majority as M4 and a significant amount as unchanged drug) and 6% was recovered in feces.11
Resistance
A Phase 2b trial (NCT03272347) evaluated three doses of ISL that were administered in combination with doravirine (DOR) in treatment-naive individuals. By Week 48, five participants who received ISL plus DOR had experienced protocol-defined virologic failure; however, all of these participants had HIV-1 RNA <80 copies/mL, and none met the criteria for resistance testing.13 No participants met the criteria for resistance testing through Week 144 of the trial.14
A Phase 3 trial (NCT04223778) evaluated a switch to a fixed-dose combination (FDC) containing DOR/ISL in participants who had virological suppression on a stable ART regimen. Through Week 96, no participants receiving DOR/ISL had virologic failure.15-17
A separate Phase 3 trial (NCT04223791) evaluated a switch to an FDC containing DOR/ISL in participants with virological suppression on bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy). Two participants receiving DOR/ISL had virologic failure through Week 96; however, no resistance was detected to either DOR or ISL in testing of plasma samples.18-20
In a Phase 3 study (NCT04233879) of a DOR/ISL FDC versus Biktarvy in treatment-naive adults, one participant receiving DOR/ISL experienced virologic failure at Week 24, which appeared to be attributed to study drug nonadherence. Genotypic testing revealed the emergence of the V106A and P225Y NNRTI mutations and the M184I NRTI mutation. Phenotypic analysis showed that the participant’s virus was resistant to DOR.21
Select Clinical Trials
On December 13, 2021, the U.S. FDA placed clinical holds on studies of ISL for HIV treatment and prevention. The FDA’s decision was based on reports of decreases in total lymphocyte and CD4 counts in some participants receiving ISL in trials. Please refer to the drug developer’s December 13, 2021 press release for more information about the ISL clinical hold.22
Subsequently, on September 20, 2022, the drug developer announced initiation of a new Phase 3 program evaluating a once-daily oral combination of DOR and a lower dose of ISL (DOR/ISL) for HIV treatment. Once-daily oral treatment studies of DOR/ISL that use doses higher than what will be studied in the new Phase 3 program remain under a partial clinical hold. Development of once-monthly oral ISL for pre-exposure prophylaxis (PrEP) will be discontinued; participants in current studies will continue to be monitored. Please refer to this September 20, 2022 press release for more information.5
Islatravir for HIV treatment
Study Identifiers: MK-8591-011; NCT03272347
Sponsor: Merck Sharp & Dohme LLC
Phase: 2b
Status: This study has been completed.
Study Purpose: The purpose of this trial was to evaluate the safety and efficacy of a regimen consisting of ISL (at three different dose levels) plus DOR and lamivudine (3TC) over 24 weeks in treatment-naive participants. After 24 weeks, investigators evaluated the safety and efficacy of ISL (at three different dose levels) plus DOR. In both cases, the active comparator group received DOR/3TC/TDF.
Study Population:
- Participants were treatment-naive adults with HIV.
- Participants had HIV RNA ≥1,000 copies/mL and CD4 counts ≥200 cells/mm3.
- Participants had no known resistance to any approved NRTI, NNRTI, PI, INSTI, or any study drug.23,24
Selected Study Results: Results published in Lancet HIV (2021) showed that treatment regimens containing ISL and DOR were highly effective in suppressing viral load levels. A high proportion of participants in all treatment groups (ISL plus DOR and comparator groups) achieved HIV RNA <50 copies/mL at Week 24 and Week 48.24 Safety results through Week 144 presented at EAC 2021 showed that rates of discontinuations due to adverse events (AEs) were low and rates of drug-related AEs with ISL plus DOR were lower than rates of drug-related AEs with the comparator regimen.14
Study Identifiers: IMAGINE-DR; MK-8591-013; NCT04564547
Sponsor: Merck Sharp & Dohme LLC
Phase: 2b
Status: This study is ongoing, but not recruiting participants. (See note below.)
Study Purpose: The purpose of this study is to evaluate the safety of oral weekly ISL plus the investigational NNRTI MK-8507 (also known as ulonivirine) based on review of accumulated safety data in participants who were virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy).
Study Population:
- Participants are adults with HIV who have been virologically suppressed on Biktarvy for at least 6 months.
- Participants have HIV RNA <50 copies/mL and CD4 counts >200 cells/mm3 at screening.25,26
Note: The developers of ISL announced in a November 18, 2021 press release that they are stopping dosing of participants in the MK-8591-013 trial. This decision was based on findings of reduced total lymphocyte and CD4 counts in study participants receiving ISL and MK-8507.26 As of December 1, 2021, the MK-8591-013 study protocol was amended and all participants have discontinued study treatment and will be switched to non-study ARV therapy. Participants who received ISL and MK-8507 will be followed for at least 6 months.25
Study Identifiers: GS-US-563-6041; NCT05052996
Sponsor: Gilead Sciences
Phase: 2
Status: This study is ongoing, but not recruiting participants.
Study Purpose: The purpose of this open-label study is to evaluate the efficacy of weekly oral ISL (2 mg) in combination with the capsid inhibitor lenacapavir (LEN) in participants who are virologically suppressed on Biktarvy.
Study Population:
- Participants are adults with HIV who have been virologically suppressed (HIV RNA <50 copies/mL) on Biktarvy for at least 24 weeks before and at screening.
- Participants have CD4 counts ≥350 cells/mm3 and have no history of virologic failure. 27,28
Selected Study Results: Results presented at CROI 2024 showed that participants switching to weekly oral ISL plus LEN maintained a high rate (94.2%) of viral suppression at Week 24, which was comparable to the rate of viral suppression in participants who remained on Biktarvy. Only one (1.9%) participant in the ISL plus LEN group had HIV RNA ≥50 copies/mL at Week 24. Notably, this participant resuppressed at Week 30 on ISL plus LEN.28
Study Identifiers: ILLUMINATE SWITCH A; MK-8591A-017; NCT04223778
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is ongoing, but not recruiting participants. (In December 2021, this study was placed on clinical hold.)
Study Purpose: The purpose of this open-label trial is to evaluate the safety and efficacy of a switch from a baseline ART regimen to an FDC containing DOR/ISL (100 mg/0.75 mg).
Study Population:
- Participants are adults with HIV who have been receiving a continuous, stable 2- or 3-drug ART regimen for at least the past 3 months.
- Participants have had viral suppression on ART with HIV RNA <50 copies/mL for at least the past 3 months.
- Participants have no history of virologic treatment failure on any past or current ART regimen.15,22
Selected Study Results: Results presented at CROI 2023 indicated that DOR/ISL was as effective as baseline ART regimens in maintaining suppression of participants’ viral load levels through 48 weeks of treatment. DOR/ISL had a safety profile that was similar to that of the comparator ART regimens.16
Additional Published Material:
- EACS 2023: Switch to fixed-dose doravirine (100 mg) with islatravir (0.75 mg) once daily in adults with HIV-1 virologically suppressed on antiretroviral therapy: Week 96 results of a randomized, open-label, Phase 3 trial
Study Identifiers: ILLUMINATE SWITCH B; MK-8591A-018; NCT04223791
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is ongoing, but not recruiting participants. (In December 2021, this study was placed on partial clinical hold.)
Study Purpose: The purpose of this trial is to evaluate the safety and efficacy of a switch from Biktarvy to an FDC containing DOR/ISL (100 mg/0.75 mg).
Study Population:
- Participants are adults with HIV who have been receiving Biktarvy for at least the past 3 months.
- Participants have had viral suppression on Biktarvy with HIV RNA <50 copies/mL for at least the past 3 months.
- Participants have no history of virologic treatment failure on any past or current ART regimen.18,22
Selected Study Results: Results presented at CROI 2023 indicated that DOR/ISL was as effective as Biktarvy in maintaining suppression of participants’ viral load levels through 48 weeks of treatment. The safety profile of DOR/ISL was similar to that of Biktarvy.19
Additional Published Material:
- EACS 2023: Switch to fixed-dose doravirine/islatravir (100/0.75 mg) once daily in adults with HIV-1 virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamide: Week 96 results of a Phase 3, randomized, double-blind, non-inferiority trial
Study Identifiers: ILLUMINATE HTE; MK-8591A-019; NCT04233216
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study has been completed.
Study Purpose: The purpose of this trial was to evaluate the safety and efficacy of ISL, DOR, and an FDC containing DOR/ISL (100 mg/0.75 mg), each compared to placebo, in heavily treatment-experienced participants.
Study Population:
- Participants were heavily treatment–experienced children and adults with HIV who had been receiving the same ART regimen for at least the past 3 months.
- Participants had resistance to at least one drug within three different ARV drug classes (NRTI, NNRTI, and either PI or INSTI) and had limited treatment options available to form a viable ART regimen.
- Participants had HIV RNA ≥500 copies/mL at screening and within the past 10 days prior to randomization.29,30
Selected Study Results: Results presented at EACS 2023 showed that virologic response from baseline to Day 8 was greatest among participants in the DOR/ISL plus ART group. Of note, the MK-8591A-019 study did not reach target enrollment, and hypothesis testing for efficacy was not performed. DOR/ISL was generally well tolerated through Week 49.30
Study Identifiers: ILLUMINATE NAIVE; MK-8591A-020; NCT04233879
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is ongoing, but not recruiting participants. (In December 2021, this study was placed on partial clinical hold.)
Study Purpose: The purpose of this trial is to evaluate the safety and efficacy of an FDC containing DOR/ISL (100 mg/0.75 mg) versus Biktarvy in treatment-naive participants.
Study Population:
- Participants are treatment-naive adults with HIV.
- Participants have no known resistance to any approved HIV-1 reverse transcriptase inhibitor or any study intervention.22,31
Selected Study Results Week 48 results presented at IAS 2023 showed that DOR/ISL was as effective as Biktarvy in suppressing viral load in treatment-naive participants. Treatment-related AEs occurred with similar frequency in both groups. More participants receiving DOR/ISL discontinued treatment due to an AE than participants receiving Biktarvy.21
Study Identifiers: MK-8591A-051; NCT05631093
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is ongoing, but not recruiting participants.
Study Purpose: The purpose of this open-label trial is to evaluate the safety and efficacy of a switch from a baseline ART regimen to an FDC containing DOR/ISL (100 mg/0.25 mg).
Study Population:
- Participants are adults with HIV who have been receiving a continuous, stable 2- or 3-drug ART regimen for at least the past 3 months.
- Participants have had viral suppression on ART with HIV RNA <50 copies/mL for at least the past 3 months and at screening.
- Participants have no history of virologic treatment failure on any past or current regimen and have no known resistance to DOR.32
Study Identifiers: MK-8591A-052; NCT05630755
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is ongoing, but not recruiting participants.
Study Purpose: The purpose of this trial is to evaluate the safety and efficacy of a switch from Biktarvy to an FDC containing DOR/ISL (100 mg/0.25 mg).
Study Population:
- Participants are adults with HIV who have been receiving Biktarvy for at least the past 3 months.
- Participants have had viral suppression on Biktarvy with HIV RNA <50 copies/mL for at least the past 3 months.
- Participants have no history of virologic treatment failure on any past or current ART regimen and have no known resistance to DOR.
- Participants have never received long-acting HIV therapy (such as cabotegravir or lenacapavir).33
Study Identifiers: MK-8591A-053; NCT05705349
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is currently recruiting participants.
Study Purpose: The purpose of this trial is to evaluate the safety and efficacy of an FDC containing DOR/ISL (100 mg/0.25 mg) as compared to Biktarvy in treatment-naive participants.
Study Population:
- Participants are treatment-naive adults with HIV.
- Participants have HIV RNA ≥500 copies/mL at screening.34
Study Identifiers: MK-8591A-054; NCT05766501
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is ongoing, but not recruiting participants.
Study Purpose: The purpose of this open-label trial is to evaluate the safety and tolerability of an FDC containing DOR/ISL (100 mg/0.25 mg) in participants who have previously been treated in earlier clinical studies with DOR/ISL (100 mg/0.75 mg).
Study Population: Participants are adults with HIV who are currently receiving DOR/ISL in one of the following clinical studies: MK-8591A-017, MK-8591A-018, MK-8591A-020, or MK-8591A-033.35
Additional trials evaluating DOR/ISL for HIV treatment have been or are being conducted, including:
- ILLUMINATE YOUTH (MK-8591A-028; NCT04295772):
A Phase 2 open-label study that evaluated DOR/ISL (100 mg/0.75 mg) as an HIV treatment in pediatric participants who were virologically suppressed on ART or treatment-naive. This study has been completed.36 - MK-8591A-033 (NCT04776252): A Phase 3 open-label rollover study evaluating the safety of DOR/ISL (100 mg/0.75 mg) in adult and pediatric participants who received DOR/ISL in a previous clinical trial. This study is ongoing, but not recruiting participants. (In December 2021, this study was placed on partial clinical hold.)22,37
Islatravir for HIV prevention
Study Identifiers: MK-8591-016; NCT04003103
Sponsor: Merck Sharp & Dohme LLC
Phase: 2a
Status: This study has been completed.
Study Purpose: The purpose of this study was to evaluate the safety, tolerability, and pharmacokinetics of two different doses of oral ISL administered once monthly to participants who were at low risk of acquiring HIV.
Study Population:
- Participants were adults without HIV who are at low risk of acquiring HIV.38
Selected Study Results: Results presented at IAS 2021 showed that both doses of once-monthly oral ISL were well-tolerated over 24 weeks. The majority of AEs that occurred were mild, and less than 1% of participants discontinued due to an AE. No serious drug-related AEs were reported.39
Additional Published Material:
Study Identifiers: IMPOWER 22; MK-8591-022; NCT04644029
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study is ongoing, but not recruiting participants. (In December 2021, this study was placed on full clinical hold.)
Study Purpose: The purpose of this study is to evaluate the safety and efficacy of oral ISL administered once monthly as PrEP in cisgender women who are at high risk of acquiring HIV.
Study Population:
- Participants are cisgender women without HIV who are at least 16 years of age and older.
- Participants have been sexually active with a male partner in the past 30 days before screening and are at high risk of acquiring HIV.22,40
Study Identifiers: IMPOWER 24; MK-8591-024; NCT04652700
Sponsor: Merck Sharp & Dohme LLC
Phase: 3
Status: This study has been completed.
Study Purpose: The purpose of this study was to evaluate the safety and efficacy of oral ISL administered once monthly as PrEP in cisgender men who have sex with men and transgender women who have sex with men and who were at high risk of acquiring HIV.
Study Population:
- Participants were cisgender men who have sex with men and transgender women who have sex with men without HIV and who were at least 16 years of age and older.
- Participants had been sexually active with male or transgender women partners in the past month before screening and were at high risk of acquiring HIV.41
Some additional studies evaluating islatravir for HIV prevention have also been conducted, including:
- MK-8591-034: A Phase 1 trial evaluating an injectable formulation of ISL. In December 2021, this study was placed on full clinical hold.22
- MK-8591-008: A Phase 1 study that evaluated a radiopaque ISL-eluting subdermal implant in healthy male and female participants at low risk of HIV infection. This study has been completed, and results are available from J Acquir Immune Defic Syndr (2023).42
Adverse Events
MK-8591-011 (NCT03272347)
In this Phase 2b study, 90 participants received ISL (in doses of 0.25 mg, 0.75 mg, or 2.25 mg) plus DOR and 3TC and 31 participants received DOR/3TC/TDF for 24 weeks (Part 1). After 24 weeks, eligible participants who were receiving ISL, DOR, and 3TC switched to a two-drug regimen of ISL plus DOR (Part 2). The Week 48 analysis found that 73% of participants who received any dose of ISL and 77% of participants who received DOR/3TC/TDF experienced at least one AE. Drug-related AEs occurred in 8% of participants who received ISL and 19% of participants who received DOR/3TC/TDF. No serious drug-related AEs were reported with ISL. Two participants who received the 2.25-mg dose of ISL discontinued treatment because of an AE; one participant experienced diarrhea, nausea, and vomiting, and one participant had HBV reactivation. Headache was more common in the combined ISL groups (11%) than in the DOR/3TC/TDF group (6%), and diarrhea was more common in the DOR/3TC/TDF group (16%) than in the combined ISL groups (7%). Most of the cases of headache and diarrhea were mild, transient, and unrelated to treatment.23,24
Results through Week 144 of the MK-8591-011 study include data from the open-label maintenance phase of the trial where all participants in the ISL plus DOR groups transitioned to the selected ISL dose of 0.75 mg plus DOR 100 mg (Part 3). The rate of drug-related AEs continued to be lower in the ISL groups (7.8%) than in the DOR/3TC/TDF group (22.6%). There were no additional participants who discontinued treatment because of an AE.14
GS-US-563-6041 (NCT05052996)
In this Phase 2 study, adults with viral suppression on Biktarvy were randomized to either weekly oral ISL plus LEN (n = 52) or continue daily oral Biktarvy (n = 52). Safety results at Week 24 showed that 76.9% of participants on ISL plus LEN and 73.1% of participants on Biktarvy had at least one AE. Treatment-related AEs, all of which were Grade 1 or 2, occurred in 17.3% of participants receiving ISL plus LEN and 5.8% of participants receiving Biktarvy. The most common treatment-related AEs with ISL plus LEN were dry mouth (3.8%) and nausea (3.8%). There were no Grade 3 or 4 treatment-related AEs in either group. Two participants discontinued ISL plus LEN because of AEs unrelated to the study drugs.27,28
ILLUMINATE SWITCH A (MK-8591A-017; NCT04223778)
In the Phase 3 ILLUMINATE SWITCH A trial, participants were randomized to continue their baseline ART (bART) regimen (n = 366) or switch to a an FDC containing DOR/ISL (n = 366). Results through Week 48 showed that DOR/ISL had a similar safety profile to that of bART. Approximately 80% of participants in the DOR/ISL arm and 70% of participants in the bART arm had at least one AE. Drug-related AEs occurred in 19.6% of DOR/ISL participants and 8.9% of bART participants. The most common drug-related AEs were insomnia (2.1% DOR/ISL; 0.3% bART), abnormal dreams (1.8% DOR/ISL; 0.3% bART), headache (1.8% DOR/ISL; 0.6% bART), nausea (1.5% DOR/ISL; 0.3% bART), pruritus (1.5% DOR/ISL; 0.0% bART), and weight increase (1.5% DOR/ISL; 0.0% bART). A drug-related serious adverse event (SAE)—paranoia—occurred in one participant receiving DOR/ISL. Five participants discontinued DOR/ISL and no participants discontinued bART due to a drug-related AE. Decreases in total lymphocyte counts and CD4 counts in the DOR/ISL group were modest and were not associated with infection-related AEs.15,16
After Week 48, all participants received open-label DOR/ISL, which was generally well tolerated. The majority of treatment-related AEs and discontinuations due to AEs that occurred after Week 48 were attributed to protocol-defined decreases in CD4 or total lymphocyte counts. Investigators determined that the decreases in CD4 and/or lymphocyte counts were generally not clinically significant. No treatment-related SAEs occurred.17
ILLUMINATE SWITCH B (MK-8591A-018; NCT04223791)
In the Phase 3 ILLUMINATE SWITCH B trial, participants were randomized to continue Biktarvy (n = 319) or switch to an FDC containing DOR/ISL (n = 322). Results through Week 48 showed that DOR/ISL had a similar safety profile to that of Biktarvy. Seventy-one percent of participants receiving DOR/ISL and 75% of participants receiving Biktarvy experienced at least one AE. Drug-related AEs occurred in 10% of DOR/ISL participants and 12% of Biktarvy participants. The most common drug-related AEs included nausea (3% DOR/ISL; 1% Biktarvy), dizziness (0% DOR/ISL; 2% Biktarvy), and myalgia (0% DOR/ISL; 2% Biktarvy). No SAEs were reported in either group. Six participants discontinued DOR/ISL, and five participants discontinued Biktarvy due to a drug-related AE. Decreases in total lymphocyte counts and CD4 counts in the DOR/ISL group were modest and were not associated with infection-related AEs.18,19
Results at Week 96 showed that a greater proportion of participants receiving DOR/ISL than participants receiving Biktarvy had treatment-related AEs and discontinued treatment due to AEs. Most of the treatment-related AEs and discontinuations due to AEs in the DOR/ISL group were attributed to protocol-defined decreases in CD4 and/or total lymphocyte counts. The decreases in CD4 and/or lymphocyte counts were not considered clinically significant. No treatment-related SAEs occurred through 96 weeks.20
ILLUMINATE HTE (MK-8591A-019; NCT04233216)
In the Phase 3 ILLUMINATE HTE study, 35 heavily treatment-experienced participants received at least one dose of study drug and completed Part 1 of the trial where ISL (n = 7), DOR (n = 14), an FDC containing DOR/ISL (n = 7), or placebo (n = 7) was added on to a baseline failing ART regimen from Day 1 to Day 8. In Part 2 of the trial, all participants received DOR/ISL plus optimized background therapy (OBT) starting on Day 8. Results at Week 49 showed that the majority of participants in the study experienced at least one AE. Drug-related AEs, most of which were attributed to DOR/ISL, occurred in 48.6% of participants. Two participants receiving DOR plus ART and one participant receiving DOR/ISL plus ART discontinued treatment due to a drug-related AE. No drug-related SAEs were reported. CD4 counts at Week 49 were only modestly different between treatment groups.29,30,43
ILLUMINATE NAIVE (MK-8591A-020; NCT04233879)
In the Phase 3 ILLUMINATE NAIVE study, treatment-naive participants received either an FDC containing DOR/ISL (n = 298) or Biktarvy (n = 299). Through Week 48, 91% of participants on DOR/ISL and 86% of participants on Biktarvy had at least one AE. Drug-related AEs occurred in 26% of DOR/ISL participants and 25% of Biktarvy participants. The most common drug-related AEs that occurred in either group included decreased lymphocyte count (11.7% DOR/ISL; 6.4% Biktarvy), headache (10.7% DOR/ISL; 10.7% Biktarvy), diarrhea (8.7% DOR/ISL; 6.4% Biktarvy), insomnia (4.4% DOR/ISL; 6.4% Biktarvy), and increased weight (3.4% DOR/ISL; 5.0% Biktarvy). There were no drug-related SAEs associated with DOR/ISL. Twenty-two participants in the DOR/ISL group and 10 participants in the Biktarvy group discontinued treatment due to an AE. The higher rate of AE-related discontinuations seen with DOR/ISL was due to protocol-required withdrawals for decreased CD4 counts or total lymphocyte counts. Infection-related AE rates were similar in both groups.21,44
MK-8591-016 (NCT04003103)
In this Phase 2a trial evaluating ISL for PrEP, participants were randomized to once monthly oral doses of ISL (60 mg [n = 97] or 120 mg [n = 97]) or placebo (n = 48). Results through Week 24 showed that approximately 60% of participants in each of the ISL groups and 67% of participants in the placebo group experienced at least one AE. The majority of AEs were mild in intensity, with the most common AEs overall being headache, diarrhea, and nausea. Drug-related AEs, all of which were mild or moderate, occurred in 9.3% of participants in the ISL 60 mg group, 15.5% of participants in the ISL 120 mg group, and 25% of participants in the placebo group. Two participants discontinued study drug because of an AE — one due to a mild foreign body sensation in the throat and one due to moderate rash and pruritus. Grade 3-4 laboratory abnormalities were uncommon.38,39
Drug Interactions
In vitro, ISL does not interact with renal or hepatic drug transporters or major enzymes involved in drug metabolism, including CYP enzymes and UGT1A1.12,45 Although ISL was shown to be a substrate of BCRP in vitro, this finding is unlikely to be clinically significant.12
Drug-drug interaction studies in adults without HIV indicate no significant interactions between ISL and either DOR or LEN.46,47 Additionally, ISL does not appear to have any clinically meaningful interaction with dolutegravir and TDF.48
The pharmacokinetics of levonorgestrel (LNG) and ethinyl estradiol (EE) were not affected when an oral contraceptive that contained these drugs was coadministered with ISL.49 Additionally, an exploratory substudy in people of childbearing potential at low risk of acquiring HIV found that once-monthly ISL appeared to have no meaningful impact on exposure to long-acting reversible contraceptives.50
Results from a two-way interaction study found that the coadministration of ISL and methadone did not significantly affect the pharmacokinetics of either drug.51
A drug-drug interaction study of a single oral dose of ISL on atorvastatin and metformin pharmacokinetics in healthy adults found that ISL has no clinically significant impact on the pharmacokinetic profiles of either atorvastatin or metformin.52
References
- National Center for Biotechnology Information. PubChem compound summary for CID 6483431, 4’-Ethynyl-2-Fluoro-2’-Deoxyadenosine. Accessed March 13, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). NIAID ChemDB, HIV Drugs in Development. Accessed March 13, 2024
- Jefferys R. The antiretroviral therapy pipeline 2023. Treatment Action Group Pipeline Report 2023. Accessed March 13, 2024
- Jefferys R. PrEP and microbicides pipeline 2023. Treatment Action Group Pipeline Report 2023. Accessed March 13, 2024
- Merck: Press Release, dated September 20, 2022. Merck to initiate new Phase 3 clinical program with lower dose of daily oral islatravir in combination with doravirine for treatment of people with HIV-1 infection. Accessed March 13, 2024
- Michailidis E, Huber AD, Ryan EM, et al. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) Inhibits HIV-1 Reverse Transcriptase with Multiple Mechanisms. J Biol Chem. 2014;289(35):24533-24548. doi:10.1074/jbc.M114.562694. Accessed March 13, 2024
- Grobler J. Efficacy of MK-8591 against diverse HIV-1 subtypes and NRTI-resistant clinical isolates. Webcast presented at: International Congress of Drug Therapy in HIV Infection (HIV Glasgow); October 28-31, 2018; Glasgow, United Kingdom. Accessed March 13, 2024
- Michailidis E, Marchand B, Kodama EN, et al. Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor. J Biol Chem. 2009;284(51):35681-35691. doi:10.1074/jbc.M109.036616. Accessed March 13, 2024
- Markowitz M, Sarafianos SG. EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine, MK-8591): a novel HIV-1 reverse transcriptase translocation inhibitor. Curr Opin HIV AIDS. 2018;13(4):294-299. doi:10.1097/COH.0000000000000467. Accessed Accessed March 13, 2024
- Matthews RP, Ankrom W, Friedman E, et al. Safety, tolerability, and pharmacokinetics of single‐ and multiple‐dose administration of islatravir (MK‐8591) in adults without HIV. Clin Transl Sci. 2021;14(5):1935-1944. doi:10.1111/cts.13048. Accessed March 13, 2024
- Matthews RP, Fillgrove KL, Patel M, et al. Characterization of the absorption, metabolism, and excretion of islatravir, an HIV nucleoside reverse transcriptase translocation inhibitor, in humans. Poster presented at: International AIDS Conference; July 29 – August 2, 2022; Montreal, Canada and Virtual. Poster EPB173. Accessed March 13, 2024
- Bleasby K, Houle R, Hafey M, et al. Islatravir is not expected to be a victim or perpetrator of drug-drug interactions via major drug-metabolizing enzymes or transporters. Viruses. 2021;13(8):1566. doi:10.3390/v13081566. Accessed March 13, 2024
- Molina JM, Yazdanpanah Y, Afani Saud A, et al. Islatravir (ISL, MK-8591) at doses of 0.25 to 2.25 mg QD, in combination with doravirine maintains viral suppression through 48 weeks in adults with HIV-1 infection. Slides presented at: IAS Conference on HIV Science; July 21-24, 2019; Mexico City, Mexico. Accessed March 13, 2024
- Molina J-M, Yazdanpanah Y, Afani A, et al. Efficacy and safety of islatravir in combination with doravirine through 144 weeks for treatment-naïve adults with HIV-1 infection in a Phase 2b trial. European AIDS Conference; October 27-30, 2021; Virtual and United Kingdom. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2021. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3 randomized, active-controlled, open-label clinical study to evaluate a switch to doravirine/islatravir (DOR/ISL) once-daily in participants with HIV-1 virologically suppressed on antiretroviral therapy. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 8, 2020. NLM Identifier: NCT04223778. Accessed March 13, 2024
- Molina J-M, Rizzardini G, Orell C, et al. Switch to DOR/ISL (100/0.75mg) QD: Week 48 results from an open-label Phase 3 trial. Conference on Retroviruses and Opportunistic Infections (CROI); February 19-22; Seattle, WA. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2023. Accessed March 13, 2024
- Molina J-M, Rizzardini G, Orell C, et al. Switch to fixed-dose doravirine (100 mg) with islatravir (0.75 mg) once daily in adults with HIV-1 virologically suppressed on antiretroviral therapy: Week 96 results of a randomized, open-label, Phase 3 trial. European AIDS Conference; October 18-21, 2023; Warsaw, Poland. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2023. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3, randomized, active-controlled, double-blind clinical study to evaluate a switch to doravirine/islatravir (DOR/ISL) once-daily in participants with HIV- 1 virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 8, 2020. NLM Identifier: NCT04223791. Accessed March 13, 2024
- Mills AM, Rizzardini G, Ramgopal M, et al. Switch to DOR/ISL (100/0.75 mg) QD from B/F/TAF: Week 48 results from a Phase 3 trial. Conference on Retroviruses and Opportunistic Infections (CROI); February 19-22; Seattle, WA. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2023. Accessed March 13, 2024
- Paredes R, Mills AM, Rizzardini G, et al. Switch to fixed-dose doravirine/islatravir (100/0.75 mg) once daily in adults with HIV-1 virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamide: Week 96 results of a Phase 3, randomized, double-blind, non-inferiority trial. European AIDS Conference; October 18-21, 2023; Warsaw, Poland. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2023. Accessed March 13, 2024
- Rockstroh JK, Paredes R, Cahn P, et al. DOR/ISL (100mg/0.75mg) QD compared to B/F/TAF as initial HIV-1 treatment: 48 week results from a double-blind Phase 3 trial. International AIDS Society (IAS) Conference on HIV Science; July 23-26, 2023; Brisbane, Australia. Conference reports for National AIDS Treatment Advocacy Project (NATAP); 2023. Accessed March 13, 2024
- Merck: Press release, dated December 13, 2021. Merck announces clinical holds on studies evaluating islatravir for the treatment and prevention of HIV-1 infection. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 2B, randomized, double-blind, active-comparator-controlled, dose-ranging clinical trial to evaluate the safety, tolerability, antiretroviral activity, and pharmacokinetics of MK-8591 given in combination with doravirine (DOR) and lamivudine (3TC) in HIV-1-infected treatment-naïve adults. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on September 1, 2017. NLM Identifier: NCT03272347. Accessed March 13, 2024
- Molina JM, Yazdanpanah Y, Afani Saud A, et al. Islatravir in combination with doravirine for treatment-naive adults with HIV-1 infection receiving initial treatment with islatravir, doravirine, and lamivudine: a phase 2b, randomised, double-blind, dose-ranging trial. The Lancet HIV. 2021;8(6):e324-e333. doi:10.1016/S2352-3018(21)00021-7. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 2b, randomized, active-controlled, double-blind, dose-ranging clinical study to evaluate a switch to islatravir (ISL) and MK-8507 once-weekly in adults with HIV-1 virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) once-daily. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on September 21, 2020. NLM Identifier: NCT04564547. Accessed March 13, 2024
- Merck: Press release, dated November 18, 2021. Merck provides update on Phase 2 clinical trial of once-weekly investigational combination of MK-8507 and islatravir for the treatment of people living with HIV-1. Accessed March 13, 2024
- Gilead Sciences. A Phase 2 randomized, open-label, active-controlled study evaluating the safety and efficacy of an oral weekly regimen of islatravir in combination with lenacapavir in virologically suppressed people with HIV. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on September 13, 2021. NLM Identifier: NCT05052996. Accessed March 13, 2024
- Colson A, Crofoot G, Ruane PJ, et al. Efficacy and safety of weekly islatravir plus lenacapavir in PWH at 24 weeks: a Phase 2 study. Conference on Retroviruses and Opportunistic Infections (CROI); March 3-6, 2024; Denver, CO. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2024. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3, randomized, clinical study in HIV-1-infected heavily treatment-experienced participants evaluating the antiretroviral activity of blinded islatravir (ISL), doravirine (DOR), and doravirine/islatravir (DOR/ISL), each compared to placebo, and the antiretroviral activity, safety, and tolerability of open-label DOR/ISL. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 15, 2020. NLM Identifier: NCT04233216. Accessed March 13, 2024
- Mngqibisa R, Khaertynova I, Kumar PN, et al. Efficacy and safety of doravirine/islatravir (100 mg/0.75 mg) once daily in heavily treatment-experienced persons with HIV-1: Week 49 results from a Phase 3 trial. European AIDS Conference; October 18-21, 2023; Warsaw, Poland. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2023. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3 randomized, active-controlled, double-blind clinical study to evaluate the antiretroviral activity, safety, and tolerability of doravirine/islatravir once-daily in HIV-1 infected treatment-naïve participants. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 15, 2020. NLM Identifier: NCT04233879. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3, randomized, active-controlled, open-label clinical study to evaluate a switch to doravirine/islatravir (DOR/ISL 100 mg/0.25 mg) once-daily in participants with HIV-1 who are virologically suppressed on antiretroviral therapy. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 18, 2022. NLM Identifier: NCT05631093. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3, randomized, active-controlled, double-blind clinical study to evaluate a switch to doravirine/islatravir (DOR/ISL 100 mg/0.25 mg) once-daily in participants with HIV-1 who are virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 18, 2022. NLM Identifier: NCT05630755. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3, randomized, active-controlled, double-blind clinical study to evaluate the antiretroviral activity, safety, and tolerability of doravirine/islatravir (DOR/ISL 100 mg/0.25 mg) once-daily in HIV-1 infected treatment-naïve participants. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 20, 2023. NLM Identifier: NCT05705349. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3 open-label clinical study of doravirine/islatravir (DOR/ISL [100 mg/0.25 mg]) once daily for the treatment of HIV-1 infection in participants who previously received DOR/ISL (100 mg/0.75 mg) QD in a Phase 3 clinical study. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on March 1, 2023. NLM Identifier: NCT05766501. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 2 clinical study to evaluate the pharmacokinetics, safety, and efficacy of doravirine/islatravir in pediatric participants with HIV-1 infection who are virologically suppressed or treatment-naïve, are less than 18 years of age, and weigh greater than or equal to 35 kg. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on March 3, 2020. NLM Identifier: NCT04295772. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3 open-label rollover clinical study of doravirine/islatravir (DOR/ISL) once-daily for the treatment of HIV-1 infection in participants who previously received DOR/ISL in a Phase 2 or Phase 3 DOR/ISL clinical study. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on February 26, 2021. NLM Identifier: NCT04776252. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 2a, double-blind, placebo-controlled study to evaluate the safety, tolerability, and pharmacokinetics of oral MK-8591 once-monthly in participants at low-risk for HIV-1 infection. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 27, 2019. NLM Identifier: NCT04003103. Accessed March 13, 2024
- Hillier S, Bekker L-G, Riddler SA, et al. Safety and pharmacokinetics of oral islatravir once monthly for HIV pre-exposure prophylaxis (PrEP): Week 24 analysis of a Phase 2a trial. IAS Conference on HIV Science; July 18-22, 2021; Virtual and Berlin, Germany. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2021. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3, randomized, active-controlled, double-blind clinical study to evaluate the efficacy and safety of oral islatravir once-monthly as preexposure prophylaxis in cisgender women at high risk for HIV-1 infection. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 23, 2020. NLM Identifier: NCT04644029. Accessed March 13, 2024
- Merck Sharp & Dohme LLC. A Phase 3, randomized, active-controlled, double-blind clinical study to evaluate the efficacy and safety of oral islatravir once-monthly as preexposure prophylaxis in cisgender men and transgender women who have sex with men, and are at high risk for HIV-1 infection. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on December 2, 2020. NLM Identifier: NCT04652700. Accessed March 13, 2024
- Matthews RP, Zang X, Barrett SE, et al. A randomized, double-blind, placebo-controlled, Phase 1 trial of radiopaque islatravir-eluting subdermal implants for pre-exposure prophylaxis against HIV-1 infection. J Acquir Immune Defic Syndr. 2023;92(4):310-316. doi:10.1097/QAI.0000000000003135. Accessed March 13, 2024
- Mngqibisa R, Khaertynova I, Kumar P, et al. Efficacy and safety of doravirine/islatravir (100 mg/0.75 mg) once daily in heavily treatment-experienced persons with HIV-1: Week 49 results from a Phase 3 trial. Abstract 497. HIV Med. 2023;24(S5):3-675. doi:10.1111/hiv.13572. Accessed March 13, 2024
- Rockstroh JK, Paredes R, Cahn P, et al. Doravirine/islatravir (100mg/0.75mg) once daily compared to bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF) as initial HIV-1 treatment: 48 week results from a double-blind Phase 3 trial. Abstract presented at: International AIDS Society (IAS) Conference on HIV Science; July 23-26; Brisbane, Australia. Abstract OALBX0102. Accessed March 13, 2024
- Matthews R, Rudd D, Fillgrove K, et al. 546. No difference in MK-8591 and doravirine pharmacokinetics after co-administration. Open Forum Infect Dis. 2018;5(Suppl 1):S203. doi:10.1093/ofid/ofy210.554. Accessed March 13, 2024
- Matthews RP, Jackson Rudd D, Fillgrove KL, et al. A Phase 1 study to evaluate the drug interaction between islatravir (MK-8591) and doravirine in adults without HIV. Clin Drug Investig. 2021;41(7):629-638. doi:10.1007/s40261-021-01046-1. Accessed March 13, 2024
- Zhang H, Mortensen E, Rhee M, et al. Evaluation of potential drug-drug interactions between islatravir and lenacapavir. Abstract presented at: Conference on Retroviruses and Opportunistic Infection (CROI); February 12-16, 2022; Virtual. Abstract 433. Accessed March 13, 2024
- Rudd DJ, Zhang S, Fillgrove KL, et al. Lack of a clinically meaningful drug interaction between the HIV-1 antiretroviral agents islatravir, dolutegravir, and tenofovir disoproxil fumarate. Clin Pharmacol Drug Dev. 2021;10(12):1432-1441. doi:10.1002/cpdd.1026. Accessed March 13, 2024
- Ankrom W, Jonathan D, Rudd D, et al. 551. MK-8591 Does Not Alter the Pharmacokinetics of the Oral Contraceptives Ethinyl Estradiol and Levonorgestrel. Open Forum Infect Dis. 2018;5(Suppl 1):S205. doi:10.1093/ofid/ofy210.559. Accessed March 13, 2024
- Pham M, Wickremasingha P, Vargo R, et al. Once-monthly islatravir has no meaningful effect on exposure to long-acting reversible contraceptives (LARCs). European AIDS Conference; October 18-21, 2023; Warsaw, Poland. Conference Reports for National AIDS Treatment Advocacy Project (NATAP); 2023. Accessed March 13, 2024
- Matthews R, Handy W, Ankrom W, et al. No pharmacokinetic interaction between islatravir and methadone. Poster presented at: International AIDS Conference; July 29-August 2, 2022; Montreal, Canada and Virutal. Poster EPB229. Accessed March 13, 2024
- McCrea J, Patel M, Liu Y, et al. Islatravir (MK-8591) has no meaningful effect on the pharmacokinetics of atorvastatin and metformin following coadministration. Abstract presented at: IAS Conference on HIV Science; Brisbane, Australia and Virtual; July 23-26, 2023. Abstract EPB0219. Accessed March 13, 2024
Last Reviewed: March 13, 2024