Drug information
| drug-audio-en-Dapivirine.mp3 |
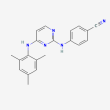
C20 H19 N5
4-[[4-(2,4,6-trimethylanilino)pyrimidin-2-yl]amino]benzonitrile
The monthly dapivirine intravaginal ring has completed Phase 3b testing as a product for HIV prevention. In the United States, a new drug application (NDA) for the monthly dapivirine ring was submitted to U.S. Food and Drug Administration (FDA); however, in December 2021, the application was withdrawn by the drug sponsor due to the unlikelihood of approval.
The monthly dapivirine ring is approved for use in South Africa, Zimbabwe, and other countries in Africa.
dapivirine
Molecular Weight: 329.4
(Compound details obtained from PubChem,1 Population Council press release,2 ClinicalTrials.gov,3,4 and International Partnership for Microbicides [IPM] press release5)
Pharmacology
Mechanism of Action
Microbicide; non-nucleoside reverse transcriptase inhibitor (NNRTI). HIV-specific topical microbicides formulated with antiretroviral (ARV) drugs, such as dapivirine, are being developed as a pre-exposure prophylaxis (PrEP) strategy to prevent the sexual transmission of HIV. ARV-based topical microbicides are designed to inhibit infection at the vaginal or rectal mucosa and directly interfere with the HIV replication cycle.6-9
Dapivirine, a substituted diarylpyrimidine derivative, irreversibly binds to and inhibits HIV reverse transcriptase, preventing the conversion of viral RNA to proviral DNA. Because of dapivirine’s tight binding and lipophilic characteristics, it may be active against both cell-free and cell-associated HIV.10,11 The dapivirine vaginal ring inhibits HIV locally, primarily within CD4 cells in the tissues of the female lower reproductive tract.12
Several different types of dapivirine-based microbicide products have been studied in clinical trials.13-18 The monthly dapivirine intravaginal ring (IVR) is the furthest along in development and has completed Phase 3b trials.3,4 Regulatory approval of the monthly dapivirine ring has been received in certain countries in Eastern and Southern Africa, including Zimbabwe and South Africa.2
Half-life (T½)
In clinical trials evaluating the dapivirine vaginal ring, the terminal elimination half-life of dapivirine was determined to be approximately 82 hours in plasma and 13 hours in cervical vaginal fluid.12
Metabolism/Elimination
An in vitro study evaluated interactions between dapivirine and drug metabolizing enzymes locally expressed in the female reproductive tract. Study results demonstrated that dapivirine is a CYP1A1 and CYP3A4 substrate; however, dapivirine undergoes minimal to no metabolism by UGT1A enzymes, UGT2B7, and Phase 2 enzymes.19
Resistance
Results from the Phase 3 Ring study (NCT01539226) showed that among participants who acquired HIV during the trial, there was no difference in the frequency of NNRTI resistance mutations detected in the dapivirine and placebo arms, with the exception of the E138A mutation (an HIV-1 subtype C polymorphism). The E138A mutation occurred in 11.7% of participants in the dapivirine group versus 1.8% of participants in the placebo group.20
In the Phase 3 ASPIRE study (NCT01617096), NNRTI resistance mutations, including E138A, occurred at a similar frequency in dapivirine and placebo seroconverters.21,22 Researchers determined that the resistance mutations seen among ASPIRE seroconverters were likely transmitted and not selected by dapivirine IVR use, and the E138A mutation was unlikely to cause a reduction in IVR efficacy.22 In addition, follow-up from ASPIRE found that the effectiveness of standard recommended NNRTI-containing ART regimens in seroconverters was not impacted by dapivirine IVR use.23
Select Clinical Trials
Dapivirine-Based IVR
Study Identifiers:(1) Ring Study; IPM 027; NCT01539226 and (2) DREAM Study; IPM 032; NCT02862171
Sponsor: International Partnership for Microbicides, Inc.
Phase: The Ring study was a Phase 3 trial, and the DREAM study was a follow-on Phase 3b trial.
Status: The Ring and DREAM studies have both been completed.
Study Purpose: The Ring study was designed to evaluate the safety and efficacy of a monthly dapivirine silicone elastomer matrix IVR (Ring-004) for the prevention of HIV infection in women. The DREAM study was an open-label follow-on study that continued to evaluate dapivirine safety and participant adherence in women who were enrolled in the Ring study.
Study Population:
- Participants in the Ring study were HIV-negative, sexually active women 18 to 45 years of age from South Africa and Uganda.
- Participants in the DREAM study were HIV-negative women who previously participated in the Ring study.3,13,20,24
Selected Study Results: Results from the Ring study published in The New England Journal of Medicine (2016) showed that the monthly dapivirine ring was safe and reduced the risk of HIV infection in women by 31% compared to placebo. Results from the follow-on DREAM study published in Lancet HIV (2021) revealed no new safety concerns with the dapivirine ring. Residual levels of dapivirine measured in used rings showed that the majority of study participants had used the ring at least some of the time. Participant adherence to monthly ring use was improved in the DREAM study compared to the Ring study.20,25
Study Identifiers: (1) ASPIRE Study; MTN-020; NCT01617096 and (2) HOPE Study; MTN-025; NCT02858037
Sponsor: International Partnership for Microbicides, Inc.
Phase: ASPIRE was a Phase 3 trial, and HOPE was a Phase 3b follow-on trial.
Status: The ASPIRE and HOPE studies have both been completed.
Study Purpose: The ASPIRE study was designed to evaluate the safety and effectiveness of a dapivirine silicone elastomer matrix IVR (Ring-004) for the prevention of HIV infection in women. HOPE was an open-label follow-on study that continued to evaluate dapivirine safety and participant adherence in women who were enrolled in ASPIRE.
Study Population:
- Participants in the ASPIRE study were HIV-negative, sexually active women 18 to 45 years of age from Malawi, South Africa, Uganda, and Zimbabwe.
- Participants in the HOPE study were HIV-negative women who previously participated in ASPIRE.4,26
Selected Study Results: Results from the ASPIRE study published in The New England Journal of Medicine (2016) demonstrated that the monthly dapivirine ring was safe and reduced the risk of HIV infection in women by 27% compared to placebo. Findings from the follow-on HOPE study presented in Lancet HIV (2021) continued to show that the dapivirine ring was well tolerated among users – the safety profile was similar to what was previously seen in other Phase 3 dapivirine vaginal ring trials. Overall, 89% of used rings had residual levels of dapivirine that indicated at least some use of the ring by participants. Adherence to the monthly dapivirine ring among HOPE study participants was good and improved over what was seen among ASPIRE study participants.21,27
Study Identifiers: DELIVER Study; MTN-042; NCT03965923
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Phase: 3b
Status: This study has been completed.
Study Purpose: The purpose of this open-label trial was to evaluate the maternal and infant safety of the monthly dapivirine ring and daily oral emtricitabine/tenofovir DF (Truvada) in pregnant women and their infants.
Study Population: Participants were pregnant women without HIV.28,29
Selected Study Results: Results presented at CROI 2023 and CROI 2024 and published in the Journal of Acquired Immune Deficiency Syndrome (2024) showed that adverse pregnancy outcomes and complications were uncommon in women who used either the monthly dapivirine ring or daily oral Truvada during the second or third trimesters of pregnancy. Adverse pregnancy outcome and complication rates were found to be similar to those seen in local communities where the study was being conducted.29-31
Study Identifiers: B-PROTECTED Study; MTN-043; NCT04140266
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Phase: 3b
Status: This study has been completed.
Study Purpose: The purpose of this open-label trial was to evaluate monthly dapivirine ring and daily oral Truvada safety and drug detection in breastfeeding mother-infant pairs.
Study Population: Participants were HIV-negative breastfeeding women and their infants (aged 6 to 12 weeks at enrollment).32,33
Selected Study Results: Results presented at CROI 2023 indicate that use of the monthly dapivirine ring in breastfeeding women appears to be safe. Although dapivirine could be detected in breastmilk, dapivirine concentrations in infant plasma were low. In the oral PrEP arm, drug concentrations in breastfeeding infants of women using daily oral Truvada were also low. There were no drug-related serious adverse events (SAEs) or Grade 3 or higher adverse events (AEs) in mothers. All adverse events in infants were unrelated to study product in both arms.33
Additional dapivirine-based IVR studies have also been completed or are ongoing or planned. These include the following trials:
- MTN-036/IPM 047 (NCT03234400) and IPM 054 (NCT05416021): A Phase 1 pharmacokinetic and safety study and a Phase 1 bioavailability study evaluating extended duration (3-month) dapivirine rings. Both studies have been completed. Results to MTN-036/IPM 047 are available from J Int AIDS Soc (2021).14,34
- MTN-030/IPM 041 (NCT02855346), MTN-044/IPM 053/CCN019 (NCT03467347), and IPM 056/CCN019B (NCT05041699): Phase 1 and 1b pharmacokinetic and safety studies assessing multipurpose prevention technology (MPT) IVRs containing dapivirine and levonorgestrel. MTN-030/IPM 041 and MTN-044/IPM 053/CCN019 have been completed and results are available from PLoS One (2024). IPM 056/CCN019B is ongoing, but not recruiting participants.15,35,36
- REACH; MTN-034 (NCT03593655): A completed Phase 2a study that evaluated the safety of and adherence to the monthly dapivirine IVR and oral Truvada in adolescent and young adult female participants.37,38 Results are available from IAS 2021, CROI 2022, and Lancet HIV (2023).
Other Dapivirine-Based Microbicide Formulations
Other dapivirine-based microbicide formulations have been studied in clinical trials. These include Phase 1 trials of a vaginal film (NCT01924091 and NCT01548560), a Phase 1 trial of a rectal gel (NCT03393468), and Phase 1/2 trials of vaginal gels (NCT00799058 and NCT00917891). A combination vaginal gel containing dapivirine and darunavir has also been studied in a Phase 1 trial (ISRCTN23353517).16-18,39–41
Adverse Events
Ring Study (NCT01539226); DREAM Study (NCT02862171)
In the Phase 3 Ring study, dapivirine IVR use in women was reported to be safe, with a similar rate of AEs in both the active drug and placebo arms. No dapivirine-related serious adverse events (SAEs) or Grade 3 or 4 AEs occurred. AEs related to dapivirine IVR included metrorrhagia, pelvic discomfort/pain, and suprapubic pain, all of which were mild in severity.20
Results from the open-label follow-on DREAM study showed a comparable safety profile to what was seen in the Ring study. Among 941 participants who enrolled in the DREAM study, approximately 66% experienced an AE. Only six participants experienced an AE that was considered to be related to the study product. There were no treatment-related SAEs.25
ASPIRE Study (NCT01617096); HOPE Study (NCT02858037)
Similarly, in the Phase 3 ASPIRE study, dapivirine IVR use in women was also reported to be safe, with a similar rate of AEs in both the active drug and placebo arms. Again, no dapivirine-related SAEs or Grade 3 or 4 AEs occurred. Dapivirine-related AEs included moderate cervicitis, urinary tract infection, headache, urinary incontinence, cervix erythema and oedema, dyspareunia, and pelvic pain.21
The open-label follow-on HOPE trial demonstrated a safety profile similar to what has been seen in other Phase 3 dapivirine IVR trials. Among 1,456 enrolled participants, no dapivirine-related SAEs or Grade 3 or higher AEs occurred. Dapivirine-related Grade 2 AEs occurred in just two participants.27
DELIVER Study (NCT03965923)
Results from the DELIVER study showed that adverse pregnancy outcomes and complications were uncommon in women who used either the monthly dapivirine ring or daily oral Truvada during the second and third trimesters of pregnancy. Adverse pregnancy outcome and complication rates were found to be similar to those seen in local communities where the study was being conducted. The most common pregnancy complications reported in both cohorts were hypertensive disorders.28-30
B-PROTECTED Study (NCT04140266)
In the B-PROTECTED study evaluating monthly dapivirine ring and daily oral Truvada use in breastfeeding mother-infant pairs, most AEs were mild or moderate in severity. Among mothers, there were no SAEs or Grade 3 or higher AEs related to the study product. Among infants, all of the AEs that occurred were unrelated to the study product.32,33
Drug Interactions
Dapivirine interactions with certain CYP and UGT enzymes, including those locally expressed in the female reproductive tract have been evaluated in in vitro studies. Data indicate that dapivirine is a CYP1A1 and CYP3A4 substrate. Additionally, dapivirine was found to be a potent inhibitor of CYP1A1; moderate inhibitor of CYP1B1, CYP2C8, CYP3A4, UGT1A1 and UGT1A4; and weak inhibitor of CYP1A2, CYP2B6, CYP2C19, UGT1A3, UGT1A6, UGT1A7, UGT1A9 and UGT2B7. Dapivirine showed no induction of CYP1A2, CYP2B6, and CYP3A4 enzymes.19
A drug-drug interaction study between dapivirine IVR (Ring-004) and miconazole nitrate (1200-mg vaginal capsule) in healthy women found that concomitant use caused local and systemic changes to levels of both drugs; however, such changes are not likely to alter the effectiveness of either drug.42
In the ASPIRE trial (NCT01617096), investigators evaluated whether the dapivirine IVR would alter the effectiveness of hormonal contraception. Among women who were receiving various forms of hormonal contraception during the study, no difference in pregnancy incidence between the dapivirine group and the placebo group was seen.43
References
- National Center for Biotechnology Information. PubChem compound summary for CID 214347, dapivirine. Accessed July 15, 2024
- Population Council: press release, dated October 4, 2022. Population Council completes asset purchase agreement from the International Partnership for Microbicides. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A follow-on, open-label trial to assess continued safety of and adherence to the dapivirine (25 mg) vaginal Ring-004 in healthy, HIV-negative women. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 25, 2016. NLM Identifier: NCT02862171. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A Phase 3B open-label follow-on trial to assess the continued safety of and adherence to a vaginal ring containing dapivirine in women. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 18, 2016. NLM Identifier: NCT02858037. Accessed July 15, 2024
- International Partnership for Microbicides (IPM): press release, dated December 9, 2021. IPM statement on US Food and Drug Administration review of dapivirine vaginal ring. Accessed July 15, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). Microbicides to block transmission of HIV. Accessed July 15, 2024
- Shattock RJ, Rosenberg Z. Microbicides: topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2(2):a007385. Accessed July 15, 2024
- Balzarini J, Van Damme L. Intravaginal and intrarectal microbicides to prevent HIV infection. CMAJ. 2005;172(4):461-464. doi:10.1503/cmaj.1041462. Accessed July 15, 2024
- Adams JL, Kashuba AD. Formulation, pharmacokinetics and pharmacodynamics of topical microbicides. Best Pract Res Clin Obstet Gynaecol. 2012;26(4):451-462. doi:10.1016/j.bpobgyn.2012.01.004. Accessed July 15, 2024
- Garg AB, Nuttall J, Romano J. The future of HIV microbicides: challenges and opportunities. Antivir Chem Chemother. 2009;19(4):143-150. doi:10.1177/095632020901900401. Accessed July 15, 2024
- Nuttall JP, Thake DC, Lewis MG, Ferkany JW, Romano JW, Mitchnick MA. Concentrations of dapivirine in the rhesus macaque and rabbit following once daily intravaginal administration of a gel formulation of [14C] dapivirine for 7 days. Antimicrob Agents Chemother. 2008;52(3):909-914. doi:10.1128/AAC.00330-07. Accessed July 15, 2024
- European Medicines Agency (EMA). Assessment report: dapivirine vaginal ring 25 mg. July 23, 2020. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A multi-centre, randomised, double-blind, placebo-controlled safety and efficacy trial of a dapivirine vaginal matrix ring in healthy HIV-negative women. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on February 21, 2012. NLM Identifier: NCT01539226. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A Phase 1, randomized pharmacokinetics and safety study of extended duration dapivirine vaginal rings. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 25, 2017. NLM Identifier: NCT03234400. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A randomized, Phase 1, open-label study in healthy HIV-negative women to evaluate the pharmacokinetics, safety and bleeding patterns associated with 90-day use of matrix vaginal rings containing 200 mg dapivirine and 320 mg levonorgestrel. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on March 9, 2018. NLM Identifier: NCT03467347. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. Comparison of the pharmacokinetics and pharmacodynamics of single dose dapivirine vaginal gel and film formulation. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on August 13, 2013. NLM Identifier: NCT01924091. Accessed July 15, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). An open label randomized Phase 1 pharmacokinetic study of dapivirine gel administered rectally to HIV-1 seronegative adults. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on January 2, 2018. NLM Identifier: NCT03393468. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A double-blind, randomized, placebo-controlled Phase I/II trial to evaluate the safety of dapivirine gel 4759, 0.05% 2.5g and dapivirine gel 4789, 0.05% 2.5g formulations as compared to the vaginal HEC-based universal placebo gel, 2.5g in healthy HIV-negative women. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on November 26, 2008. NLM Identifier: NCT00799058. Accessed July 15, 2024
- Valicherla GR, Graebing P, Zhang J, et al. Investigating the Contribution of Drug-metabolizing enzymes in drug-drug interactions of dapivirine and miconazole. Pharmaceutics. 2021;13(12):2193. doi:10.3390/pharmaceutics13122193. Accessed July 15, 2024
- Nel A, van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133-2143. doi:10.1056/NEJMoa1602046. Accessed July 15, 2024
- Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121-2132. doi:10.1056/NEJMoa1506110. Accessed July 15, 2024
- Parikh UM, Penrose KJ, Heaps AL, et al. HIV‐1 drug resistance among individuals who seroconverted in the ASPIRE dapivirine ring trial. J Int AIDS Soc. 2021;24(11):e25833. doi:10.1002/jia2.25833. Accessed July 15, 2024
- Riddler S, Balkus J, Mellors J, et al. NNRTI-containing ART is effective for dapivirine ring breakthrough HIV-1 infection. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 13-16, 2017; Seattle, WA. Abstract 952. Accessed July 15, 2024
- Rosenberg Z. Dapivirine ring: the roadmap to licensure. Slides presented at: MTN Regional Meeting; October 4-8, 2015; Cape Town, South Africa. Accessed July 15, 2024
- Nel A, Niekerk N van, Baelen BV, et al. Safety, adherence, and HIV-1 seroconversion among women using the dapivirine vaginal ring (DREAM): an open-label, extension study. The Lancet HIV. 2021;8(2):e77-e86. doi:10.1016/S2352-3018(20)30300-3. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A multi-center, randomized, double-blind, placebo-controlled Phase 3 safety and effectiveness trial of a vaginal matrix ring containing dapivirine for the prevention of HIV-1 infection in women. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 8, 2012. NLM Identifier: NCT01617096. Accessed July 15, 2024
- Baeten JM, Palanee-Phillips T, Mgodi NM, et al. Uptake and use of a vaginal ring containing dapivirine for HIV-1 prevention in African women: an open-label extension study. Lancet HIV. 2021;8(2):e87-e95. doi:10.1016/S2352-3018(20)30304-0. Accessed July 15, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). Phase 3b, randomized, open label safety trial of dapivirine vaginal ring and oral Truvada® use in pregnancy. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on May 24, 2019. NLM Identifier: NCT03965923. Accessed July 15, 2024
- Bunge KE, Balkus J, Mhlanga F, et al. DELIVER: a safety study of a dapivirine vaginal ring and oral PrEP during pregnancy. Webcast presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 19-22, 2023; Seattle, WA. Accessed July 15, 2024
- Mhlanga F, Bunge KE, Fairlie L, et al. Safety of dapivirine vaginal ring and oral PrEP for HIV prevention in the second trimester. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); March 3-6, 2024; Denver, CO. Abstract 168. Accessed July 15, 2024
- Bunge K, Balkus JE, Fairlie L, et al. DELIVER: a safety study of a dapivirine vaginal ring and oral PrEP for the prevention of HIV during pregnancy. J Acquir Immune Defic Syndr. 2024;95(1):65-73. doi:10.1097/QAI.0000000000003312. Accessed July 15, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). Phase 3B, randomized, open-label, safety, and drug detection study of dapivirine vaginal ring and oral Truvada® in breastfeeding mother-infant pairs. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on October 24, 2019. NLM Identifier: NCT04140266. Accessed July 15, 2024
- Owor M, Noguchi L, Horne E, et al. Dapivirine vaginal ring safety and drug detection in breastfeeding mother-infant pairs. Poster presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 19-22, 2023; Seattle, WA. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A Phase I, open-label, randomized, crossover trial to investigate the relative bioavailability of the 25 mg dapivirine vaginal Ring-004 inserted every 30 days and 100 mg DPV Ring-008 inserted for 90 days in healthy female participants. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on April 14, 2022. NLM Identifier: NCT05416021. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A randomized, double-blind, Phase 1b study in healthy HIV-negative women to evaluate the pharmacokinetics, safety, and bleeding patterns associated with 90-day use of core-sheath vaginal rings releasing dapivirine and levonorgestrel. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on August 24, 2021. NLM Identifier: NCT05041699. Accessed July 15, 2024
- National Institute of Allergy and Infectious Diseases (NIAID). A Phase 2a crossover trial evaluating the safety of and adherence to a vaginal matrix ring containing dapivirine and oral emtricitabine/tenofovir disoproxil fumarate in an adolescent and young adult female population. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on July 10, 2018. NLM Identifier: NCT03593655. Accessed July 15, 2024
- Microbicide Trials Network (MTN) website. About the REACH Study (MTN-034). Accessed July 15, 2024
- International Partnership for Microbicides, Inc. Assessing the Safety of Dapivirine Gel and Film Formulations. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on March 1, 2012. NLM Identifier: NCT01548560. Accessed July 15, 2024
- International Partnership for Microbicides, Inc. A double-blind, randomized, placebo-controlled Phase I/II study to evaluate the safety and acceptability of dapivirine gel 4759, 0.05%, 2.5g, a vaginal microbicide, conducted using daily monitored adherence in healthy, HIV-negative women. In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Registered on June 8, 2009. NLM Identifier: NCT00917891. Accessed July 15, 2024
- ISRCTN Registry. ISRCTN number: ISRCTN23353517; Assessing the safety, pharmacokinetics and pharmacodynamics of single and 14 days dosing with two vaginal microbicide formulations containing either darunavir, or dapivirine and darunavir. Accessed July 15, 2024
- Nel A, Haazen W, Russell M, Nuttall J, Van Niekerk N, Treijtel N. Drug-drug Interactions between the Dapivirine Vaginal Ring (Ring-004) and Miconazole Nitrate Vaginal Capsule (Gyno-Daktarin®). AIDS Research and Human Retroviruses. 2014;30(S1):A144-A144. doi:10.1089/aid.2014.5291.abstract. Accessed July 15, 2024
- Balkus J, Palanee-Phillips T, Siva S, et al. Dapivirine ring use does not diminish the effectiveness of hormonal contraception. Abstract presented at: Conference on Retroviruses and Opportunistic Infections (CROI); February 13–16, 2017; Seattle, WA. Abstract 88. Accessed July 15, 2024
Last Reviewed: July 15, 2024