Figure 1. Recommended Immunization Schedule for Children with HIV Infection Aged 0 through 18 Years; United States, 2019
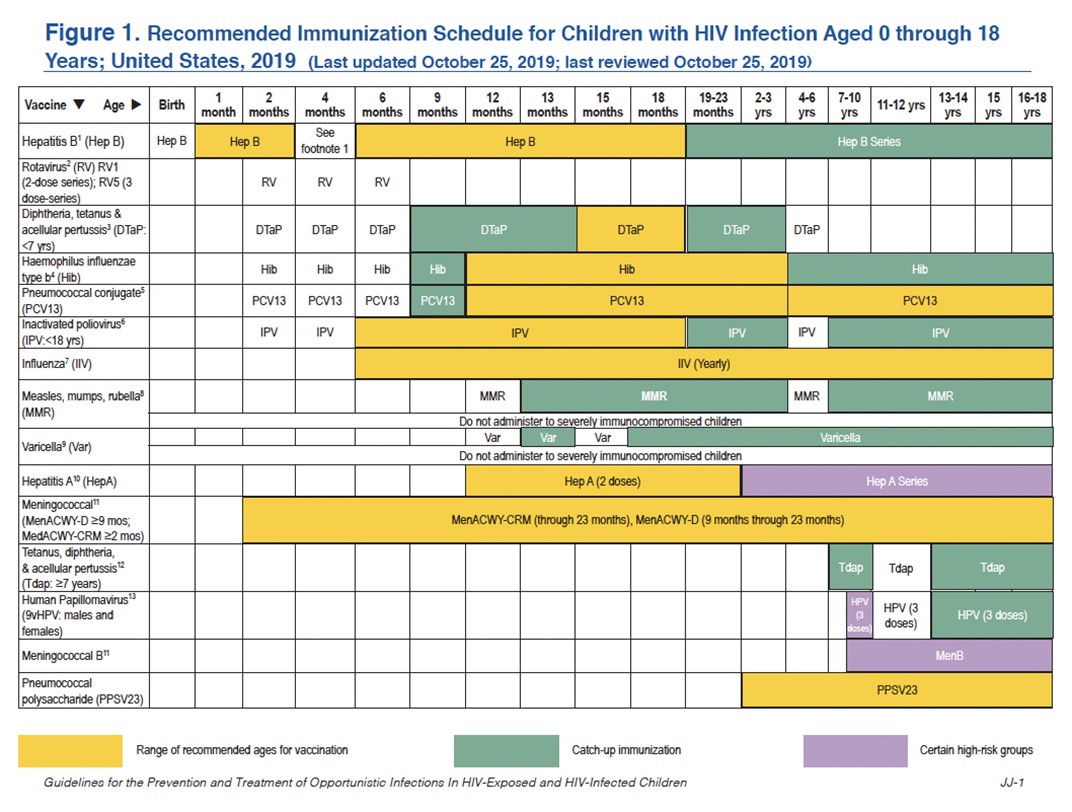
This schedule summarizes recommendations for routine administration of vaccines for children with HIV aged 0 through 18 years and indicates the recommended ages for vaccine administration in this population for childhood vaccines licensed in the United States. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. Licensed combination vaccines may be used whenever any component of the combination is indicated, when other components of the vaccine are not contraindicated, and if approved by the Food and Drug Administration for that dose of the series. The combination measles, mumps, rubella, and varicella vaccine (MMRV) is an exception; in many circumstances measles, mumps, and rubella vaccine (MMR) and varicella vaccine (Var) should be administered to persons with immunocompetent HIV infection. MMRV is contraindicated in immunocompetent HIV infection. Providers should consult the relevant Advisory Committee on Immunization Practices (ACIP) statement for detailed recommendations. Clinically significant adverse events that follow immunization should be reported to the Vaccine Adverse Event Reporting System (VAERS). Guidance about how to obtain and complete a VAERS form is available at the VAERS website or telephone 1-800-822-7967.
These recommendations should also be used for children perinatally exposed to HIV who are awaiting laboratory confirmation that they have not contracted HIV; in the United States, HIV can be reasonably excluded in most HIV-exposed infants by 4 weeks of age (see the Department of Health and Human Services [HHS] Pediatric Antiretroviral Guidelines).
1. Hepatitis B Vaccine (HepB)
Minimum Age: Birth
At Birth:
- Administer monovalent HepB to newborns before hospital discharge. Normal-weight infants of mothers who are hepatitis B surface antigen (HBsAg)-negative should receive HepB within 24 hours of birth or at discharge, whichever comes first.
- If mother is HBsAg-positive, administer HepB and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hours after birth.
- If mother’s HBsAg status is unknown, administer HepB within 12 hours after birth. Determine mother’s HBsAg status as soon as possible and, if HBsAg-positive, administer HBIG as soon as possible. If the infant weighs <2,000 grams at birth, do not wait more than 12 hours after birth to administer HBIG. If the infant weighs ≥2,000 grams at birth, do not wait more than 7 days to administer HBIG.
After the Birth Dose:
- The HepB series should be completed with either monovalent HepB or a combination vaccine containing HepB. The second dose should be administered at age 1 through 2 months.
- Monovalent HepB should be used for doses administered before age 6 weeks. The final dose should be administered no earlier than age 24 weeks. Infants who did not receive a HepB birth dose should receive three doses of a HepB- containing vaccine on an age-appropriate schedule.
Four-Month Dose:
- It is permissible to administer four doses of HepB when combination vaccines are administered after the birth dose. If monovalent HepB is used for doses after the birth dose, a dose at age 4 months is not needed.
Adolescents:
- Administer the series to those who were not previously vaccinated.
Post-Vaccination:
- Infants born to HBsAg-positive mothers should be tested for HBsAg and the antibody to HBsAg (anti- HBs) after completing at least three doses of a licensed HepB series, at ages 9 months through 12 months (generally at the next well-child visit).
- Testing for anti-HBs is also recommended for children and adolescents with HIV and should be performed 1 to 2 months after administration of the last dose of the vaccine series using a method that allows determination of a protective level of anti-HBs (≥10 mIU/mL).
- Children and adolescents with anti-HBs <10 mIU/mL after the primary schedule should receive a second series, followed by anti-HBs testing 1 to 2 months after the third dose.
Booster Dose:
- In children and adolescents with HIV, the need for booster doses has not been determined. Annual anti-HBs testing and booster doses when anti-HBs levels decline to <10 mIU/mL should be considered in individuals with ongoing risk for exposure (see https://www.cdc.gov/mmwr/pdf/rr/rr5416.pdf).
2. Rotavirus Vaccine (RV)
Minimum Age: 6 Weeks
- Practitioners should consider the potential risks and benefits of administering RV to infants with known or suspected altered immunocompetence. Consultation with an immunologist or infectious disease specialist is advised, particularly for infants with HIV infection who have a low CD4 T lymphocyte (CD4) cell percentage or count. Limited safety and efficacy data are available for the administration of RVs to infants who are potentially immunocompromised, including those with HIV infection. However, the following considerations support vaccination of infants who are HIV exposed or HIV infected:
- In infants born to mothers with HIV, an HIV diagnosis may not be established before the age of the first RV dose (≤2% of infants with perinatal HIV exposure in the United States will eventually be determined to have HIV infection), and
- Vaccine strains of rotavirus are considerably attenuated.
- RV can be administered to infants with HIV irrespective of CD4 count and percentage.
- The maximum age for the first dose in the RV series is 14 weeks and 6 days; for the final dose in the series, it is 8 months and 0 days. Vaccination should not be initiated for infants aged ≥15 weeks and 0 days.
- If Rotarix® is administered at ages 2 months and 4 months, a dose at age 6 months is not indicated.
3. Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine (DTaP)
Minimum Age: 6 Weeks
- DTaP is recommended at ages 2, 4, 6, and 15 months through 18 months, and ages 4 through 6 years.
- The fourth dose may be administered as early as age 12 months, provided that at least 6 months have elapsed since the third dose.
4. Haemophilus Influenzae Type B (Hib) Conjugate Vaccine
Minimum Age: 6 Weeks
- If PRP-OMP (PedvaxHIB®) is administered at ages 2 and 4 months, a dose at age 6 months is not indicated.
- Children aged 12 months through 59 months who have received either no doses or only one dose of Hib vaccine before 12 months of age, should receive two additional doses of Hib vaccine 8 weeks apart; children who received two or more doses of Hib vaccine before 12 months of age should receive one additional dose.
- One dose of Hib vaccine should be administered to persons aged 5 years through 18 years if they have not received a primary series and booster dose or at least one dose of Hib vaccine after 14 months of age.
5. Pneumococcal Conjugate Vaccine (13-valent) (PCV13) and Pneumococcal Polysaccharide Vaccine (23-valent) (PPSV23)
Minimum Age: 6 Weeks for PCV13; 2 Years for PPSV23
- A PCV series begun with 7-valent PCV (PCV7) should be completed with PCV13. For incompletely vaccinated children aged 24 months through 71 months, administer two doses of PCV13 ≥8 weeks apart. Children who have previously received three PCV13 doses need only one dose.
- A single dose of PCV13 should be routinely administered to children with HIV infection aged 6 years through 18 years who did not previously receive a dose of PCV13 before age 6 years. The dose should be administered ≥8 weeks after the previous dose of PCV.
- Children aged ≥2 years also should receive PPSV23 8 weeks after their last PCV dose. A second dose of PPSV23 should be administered 5 years after the first dose of PPSV23.
6. Inactivated Polio Vaccine (IPV)
Minimum Age: 6 Weeks
- If four or more doses are administered prior to age 4 years, an additional dose should be administered at age 4 years through 6 years.
- The final dose in the series should be administered on or after the child’s fourth birthday and at least 6 months after the previous dose.
7. Inactivated Influenza Vaccine (IIV)
- Administer annually to children with HIV aged 6 months through 18 years and to all their eligible close contacts (including household members). IIV is recommended for children with HIV.
- Administer two doses (separated by at least 4 weeks) to children aged <9 years per current influenza vaccine recommendations.
8. Measles, Mumps, and Rubella Vaccine (MMR)
Minimum Age: 12 Months
- Two doses of MMR vaccine for all individuals with HIV infection aged ≥12 months who do not have evidence of current severe immunosuppression as defined by the Advisory Committee on Immunization Practices (see https://www.cdc.gov/mmwr/pdf/rr/rr6204.pdf).
- The first dose should be administered at age 12 months through 15 months and the second dose at age 4 years through 6 years (or as early as 28 days after the first dose).
- Individuals with perinatal HIV infection who were vaccinated prior to establishment of effective antiretroviral therapy (ART) should receive two appropriately spaced doses of MMR vaccine once effective ART has been established and there is no evidence of current severe immunosuppression as defined by ACIP (see https://www.cdc.gov/mmwr/pdf/rr/rr6204.pdf).
9. Varicella Vaccine
Minimum Age: 12 Months
- Limited data are available on safety and immunogenicity of varicella vaccine in children with HIV infection aged 1 year through 8 years in Centers for Disease and Control Prevention immunologic categories 1 and 2 (CD4 percentages ≥15%) and clinical categories N, A, and B.
- Single-antigen varicella vaccine should be considered for children and adolescents with HIV infection with CD4 percentages ≥15%.
- Eligible children should receive two doses 3 months apart.
- MMRV vaccine has not been studied in children or adolescents with HIV infection and should not be substituted for single-antigen varicella vaccine.
10. Hepatitis A Vaccine (HepA)
Minimum Age: 12 Months
- Administer to all children aged 12 months through 23 months. The two doses in the series should be administered ≥6 months apart.
- Children who are not fully vaccinated by age 2 years can be vaccinated at subsequent well-child visits.
- HepA is also recommended for persons aged ≥24 months who live in areas where vaccination programs target older children, who are at increased risk of infection, or for whom immunity against hepatitis A is desired—see Prevention of Hepatitis A Through Active or Passive Immunization: Recommendations of the ACIP.
11. Meningococcal Vaccine
Minimum Ages: 2 months for meningococcal conjugate vaccine (Menveo) (MenACWY-CRM); 9 months for meningococcal conjugate vaccine (Menactra) (MenACWY-D); 16 years for serogroup B meningococcal (Men B) vaccines, including (Bexsero) (MenB-4C) and (Trumenba) (MenB-FHbp); 10 years for HIV infection plus another high-risk condition for serogroup B meningococcal (MenB) vaccines, including (Bexsero) (MenB-4C) and (Trumenba) (MenB-FHbp)
Meningococcal ACWY Conjugate Vaccines:
- Menveo
- Children who initiate vaccination at 8 weeks: administer doses at 2, 4, 6 and 12 months of age.
- Unvaccinated children who initiate vaccination at 7 through 23 months: administer two doses, with the second dose ≥12 weeks after the first dose AND after the first birthday.
- Children aged ≥24 months who have not received a complete series: administer two primary doses ≥8 weeks apart.
- Menactra
- Children aged ≥24 months who have not received a complete series: administer two primary doses ≥8 weeks apart. If Menactra is administered to a child with asplenia (including sickle cell disease), do not administer Menactra until age 2 years and at least 4 weeks after the completion of all PCV13 doses.
Meningococcal B Vaccines
Clinical Discretion:
- Young adults aged 16 years through 23 years (preferred age range is 16 years through 18 years) may be vaccinated with either a two-dose series of Bexsero or a three-dose series of Trumenba vaccine to provide short-term protection against most strains of serogroup B meningococcal disease. The two MenB vaccines are not interchangeable; the same vaccine product must be used for all doses.
- For booster doses among persons with high-risk conditions, refer to Prevention and Control of Meningococcal Disease.
12. Tetanus and Diphtheria Toxoids and Acellular Pertussis Vaccine (Tdap)
Minimum Age: 7 Years
- Children aged 7 years through 10 years who are not fully immunized against pertussis (i.e., have not received four or five doses of pertussis vaccine with the last dose administered on or after their fourth birthday) should receive a dose of Tdap after their seventh birthday. If Tdap is administered at age 7 years through 10 years, another dose of Tdap should be administered at 11 through 12 years of age.
- Individuals aged 11 through 18 years who have not received Tdap should receive a dose of the vaccine followed by tetanus and diphtheria vaccine (Td) booster doses every 10 years thereafter.
- Administer one dose of Tdap vaccine to pregnant girls and women during each pregnancy (preferred early during 27 through 36 weeks gestation) regardless of the time since prior Td or Tdap vaccination.
13. 9-Valent Human Papillomavirus Vaccine (9vHPV)
Minimum Age: 9 years
Note: Because 9vHPV is not a live virus vaccine, it can be administered to individuals who are immunosuppressed because of disease or medication, including those with HIV infection. However, the immune response and vaccine efficacy in immunosuppressed individuals may be less than in immunocompetent individuals.
- HPV vaccines are most effective for both males and females when given before exposure to HPV through sexual contact.
- Administer the first dose at age 11 or 12 years.
- Administer the second dose 1 to 2 months after the first dose and the third dose 6 months after the first dose (≥24 weeks after the first dose).
- Administer the series at ages 13 through 26 years if not previously vaccinated.
- HPV can be administered in a 3-dose series to individuals beginning at age 9 years.
Figures
Figure 1. Recommended Immunization Schedule for Children and Adolescents with HIV Aged 0 Through 18 Years; United States, 2024
| Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunization | Birth | 1 mo. | 2 mo. | 4 mo. | 6 mo. | 9 mo. | 12 mo. | 13 mo. | 15 mo. | 18 mo. |
| Respiratory syncytial virus (RSV-mAb [nirsevimab]) | ||||||||||
| Hepatitis B (HepB) | ||||||||||
| Rotavirus (RV) RV1 (2-dose series); RV5 (3-dose series) | ||||||||||
| Diphtheria, tetanus, and acellular pertussis (DTaP: <7 yrs) | ||||||||||
| Haemophilus influenzae type b (Hib) | ||||||||||
Legend ∎ Range of recommended ages for vaccination ∎ Catch-up immunization ∎ Certain high-risk groups ∎ Recommended vaccination based on shared clinical decision-making | ||||||||||
This schedule summarizes recommendations for routine administration of vaccines for children with HIV aged 0 through 18 years and indicates the recommended ages for vaccine administration in this population for childhood vaccines licensed in the United States. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. Licensed combination vaccines may be used whenever any component of the combination is indicated, when other components of the vaccine are not contraindicated, and if approved by the Food and Drug Administration for that dose of the series. The combination measles, mumps, rubella, and varicella vaccine (MMRV) is an exception; in many circumstances measles, mumps, and rubella vaccine (MMR) and varicella vaccine (Var) should be administered to persons with immunocompetent HIV infection. MMRV is contraindicated in immunocompetent HIV infection. Providers should consult the relevant Advisory Committee on Immunization Practices (ACIP) statement for detailed recommendations. Clinically significant adverse events that follow immunization should be reported to the Vaccine Adverse Event Reporting System (VAERS). Guidance about how to obtain and complete a VAERS form is available at the VAERS website or telephone 1-800-822-7967.
These recommendations should also be used for children perinatally exposed to HIV who are awaiting laboratory confirmation that they have not contracted HIV; in the United States, HIV can be reasonably excluded in most HIV-exposed infants by 4 weeks of age (see the Department of Health and Human Services [HHS] Pediatric Antiretroviral Guidelines).
1. Hepatitis B Vaccine (HepB)
Minimum Age: Birth
At Birth:
- Administer monovalent HepB to newborns before hospital discharge. Normal-weight infants of mothers who are hepatitis B surface antigen (HBsAg)-negative should receive HepB within 24 hours of birth or at discharge, whichever comes first.
- If mother is HBsAg-positive, administer HepB and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hours after birth.
- If mother’s HBsAg status is unknown, administer HepB within 12 hours after birth. Determine mother’s HBsAg status as soon as possible and, if HBsAg-positive, administer HBIG as soon as possible. If the infant weighs <2,000 grams at birth, do not wait more than 12 hours after birth to administer HBIG. If the infant weighs ≥2,000 grams at birth, do not wait more than 7 days to administer HBIG.
After the Birth Dose:
- The HepB series should be completed with either monovalent HepB or a combination vaccine containing HepB. The second dose should be administered at age 1 through 2 months.
- Monovalent HepB should be used for doses administered before age 6 weeks. The final dose should be administered no earlier than age 24 weeks. Infants who did not receive a HepB birth dose should receive three doses of a HepB- containing vaccine on an age-appropriate schedule.
Four-Month Dose:
- It is permissible to administer four doses of HepB when combination vaccines are administered after the birth dose. If monovalent HepB is used for doses after the birth dose, a dose at age 4 months is not needed.
Adolescents:
- Administer the series to those who were not previously vaccinated.
Post-Vaccination:
- Infants born to HBsAg-positive mothers should be tested for HBsAg and the antibody to HBsAg (anti- HBs) after completing at least three doses of a licensed HepB series, at ages 9 months through 12 months (generally at the next well-child visit).
- Testing for anti-HBs is also recommended for children and adolescents with HIV and should be performed 1 to 2 months after administration of the last dose of the vaccine series using a method that allows determination of a protective level of anti-HBs (≥10 mIU/mL).
- Children and adolescents with anti-HBs <10 mIU/mL after the primary schedule should receive a second series, followed by anti-HBs testing 1 to 2 months after the third dose.
Booster Dose:
- In children and adolescents with HIV, the need for booster doses has not been determined. Annual anti-HBs testing and booster doses when anti-HBs levels decline to <10 mIU/mL should be considered in individuals with ongoing risk for exposure (see https://www.cdc.gov/mmwr/pdf/rr/rr5416.pdf).
2. Rotavirus Vaccine (RV)
Minimum Age: 6 Weeks
- Practitioners should consider the potential risks and benefits of administering RV to infants with known or suspected altered immunocompetence. Consultation with an immunologist or infectious disease specialist is advised, particularly for infants with HIV infection who have a low CD4 T lymphocyte (CD4) cell percentage or count. Limited safety and efficacy data are available for the administration of RVs to infants who are potentially immunocompromised, including those with HIV infection. However, the following considerations support vaccination of infants who are HIV exposed or HIV infected:
- In infants born to mothers with HIV, an HIV diagnosis may not be established before the age of the first RV dose (≤2% of infants with perinatal HIV exposure in the United States will eventually be determined to have HIV infection), and
- Vaccine strains of rotavirus are considerably attenuated.
- RV can be administered to infants with HIV irrespective of CD4 count and percentage.
- The maximum age for the first dose in the RV series is 14 weeks and 6 days; for the final dose in the series, it is 8 months and 0 days. Vaccination should not be initiated for infants aged ≥15 weeks and 0 days.
- If Rotarix® is administered at ages 2 months and 4 months, a dose at age 6 months is not indicated.
3. Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine (DTaP)
Minimum Age: 6 Weeks
- DTaP is recommended at ages 2, 4, 6, and 15 months through 18 months, and ages 4 through 6 years.
- The fourth dose may be administered as early as age 12 months, provided that at least 6 months have elapsed since the third dose.
4. Haemophilus Influenzae Type B (Hib) Conjugate Vaccine
Minimum Age: 6 Weeks
- If PRP-OMP (PedvaxHIB®) is administered at ages 2 and 4 months, a dose at age 6 months is not indicated.
- Children aged 12 months through 59 months who have received either no doses or only one dose of Hib vaccine before 12 months of age, should receive two additional doses of Hib vaccine 8 weeks apart; children who received two or more doses of Hib vaccine before 12 months of age should receive one additional dose.
- One dose of Hib vaccine should be administered to persons aged 5 years through 18 years if they have not received a primary series and booster dose or at least one dose of Hib vaccine after 14 months of age.
5. Pneumococcal Conjugate Vaccine (13-valent) (PCV13) and Pneumococcal Polysaccharide Vaccine (23-valent) (PPSV23)
Minimum Age: 6 Weeks for PCV13; 2 Years for PPSV23
- A PCV series begun with 7-valent PCV (PCV7) should be completed with PCV13. For incompletely vaccinated children aged 24 months through 71 months, administer two doses of PCV13 ≥8 weeks apart. Children who have previously received three PCV13 doses need only one dose.
- A single dose of PCV13 should be routinely administered to children with HIV infection aged 6 years through 18 years who did not previously receive a dose of PCV13 before age 6 years. The dose should be administered ≥8 weeks after the previous dose of PCV.
- Children aged ≥2 years also should receive PPSV23 8 weeks after their last PCV dose. A second dose of PPSV23 should be administered 5 years after the first dose of PPSV23.
6. Inactivated Polio Vaccine (IPV)
Minimum Age: 6 Weeks
- If four or more doses are administered prior to age 4 years, an additional dose should be administered at age 4 years through 6 years.
- The final dose in the series should be administered on or after the child’s fourth birthday and at least 6 months after the previous dose.
7. Inactivated Influenza Vaccine (IIV)
- Administer annually to children with HIV aged 6 months through 18 years and to all their eligible close contacts (including household members). IIV is recommended for children with HIV.
- Administer two doses (separated by at least 4 weeks) to children aged <9 years per current influenza vaccine recommendations.
8. Measles, Mumps, and Rubella Vaccine (MMR)
Minimum Age: 12 Months
- Two doses of MMR vaccine for all individuals with HIV infection aged ≥12 months who do not have evidence of current severe immunosuppression as defined by the Advisory Committee on Immunization Practices (see https://www.cdc.gov/mmwr/pdf/rr/rr6204.pdf).
- The first dose should be administered at age 12 months through 15 months and the second dose at age 4 years through 6 years (or as early as 28 days after the first dose).
- Individuals with perinatal HIV infection who were vaccinated prior to establishment of effective antiretroviral therapy (ART) should receive two appropriately spaced doses of MMR vaccine once effective ART has been established and there is no evidence of current severe immunosuppression as defined by ACIP (see https://www.cdc.gov/mmwr/pdf/rr/rr6204.pdf).
9. Varicella Vaccine
Minimum Age: 12 Months
- Limited data are available on safety and immunogenicity of varicella vaccine in children with HIV infection aged 1 year through 8 years in Centers for Disease and Control Prevention immunologic categories 1 and 2 (CD4 percentages ≥15%) and clinical categories N, A, and B.
- Single-antigen varicella vaccine should be considered for children and adolescents with HIV infection with CD4 percentages ≥15%.
- Eligible children should receive two doses 3 months apart.
- MMRV vaccine has not been studied in children or adolescents with HIV infection and should not be substituted for single-antigen varicella vaccine.
10. Hepatitis A Vaccine (HepA)
Minimum Age: 12 Months
- Administer to all children aged 12 months through 23 months. The two doses in the series should be administered ≥6 months apart.
- Children who are not fully vaccinated by age 2 years can be vaccinated at subsequent well-child visits.
- HepA is also recommended for persons aged ≥24 months who live in areas where vaccination programs target older children, who are at increased risk of infection, or for whom immunity against hepatitis A is desired—see Prevention of Hepatitis A Through Active or Passive Immunization: Recommendations of the ACIP.
11. Meningococcal Vaccine
Minimum Ages: 2 months for meningococcal conjugate vaccine (Menveo) (MenACWY-CRM); 9 months for meningococcal conjugate vaccine (Menactra) (MenACWY-D); 16 years for serogroup B meningococcal (Men B) vaccines, including (Bexsero) (MenB-4C) and (Trumenba) (MenB-FHbp); 10 years for HIV infection plus another high-risk condition for serogroup B meningococcal (MenB) vaccines, including (Bexsero) (MenB-4C) and (Trumenba) (MenB-FHbp)
Meningococcal ACWY Conjugate Vaccines:
- Menveo
- Children who initiate vaccination at 8 weeks: administer doses at 2, 4, 6 and 12 months of age.
- Unvaccinated children who initiate vaccination at 7 through 23 months: administer two doses, with the second dose ≥12 weeks after the first dose AND after the first birthday.
- Children aged ≥24 months who have not received a complete series: administer two primary doses ≥8 weeks apart.
- Menactra
- Children aged ≥24 months who have not received a complete series: administer two primary doses ≥8 weeks apart. If Menactra is administered to a child with asplenia (including sickle cell disease), do not administer Menactra until age 2 years and at least 4 weeks after the completion of all PCV13 doses.
Meningococcal B Vaccines
Clinical Discretion:
- Young adults aged 16 years through 23 years (preferred age range is 16 years through 18 years) may be vaccinated with either a two-dose series of Bexsero or a three-dose series of Trumenba vaccine to provide short-term protection against most strains of serogroup B meningococcal disease. The two MenB vaccines are not interchangeable; the same vaccine product must be used for all doses.
- For booster doses among persons with high-risk conditions, refer to Prevention and Control of Meningococcal Disease.
12. Tetanus and Diphtheria Toxoids and Acellular Pertussis Vaccine (Tdap)
Minimum Age: 7 Years
- Children aged 7 years through 10 years who are not fully immunized against pertussis (i.e., have not received four or five doses of pertussis vaccine with the last dose administered on or after their fourth birthday) should receive a dose of Tdap after their seventh birthday. If Tdap is administered at age 7 years through 10 years, another dose of Tdap should be administered at 11 through 12 years of age.
- Individuals aged 11 through 18 years who have not received Tdap should receive a dose of the vaccine followed by tetanus and diphtheria vaccine (Td) booster doses every 10 years thereafter.
- Administer one dose of Tdap vaccine to pregnant adolescents during each pregnancy (preferred early during 27 through 36 weeks gestation) regardless of the time since prior Td or Tdap vaccination.
13. 9-Valent Human Papillomavirus Vaccine (9vHPV)
Minimum Age: 9 years
Note: Because 9vHPV is not a live virus vaccine, it can be administered to individuals who are immunosuppressed because of disease or medication, including those with HIV infection. However, the immune response and vaccine efficacy in immunosuppressed individuals may be less than in immunocompetent individuals.
- HPV vaccines are most effective for both males and females when given before exposure to HPV through sexual contact.
- Administer the first dose at age 11 or 12 years.
- Administer the second dose 1 to 2 months after the first dose and the third dose 6 months after the first dose (≥24 weeks after the first dose).
- Administer the series at ages 13 through 26 years if not previously vaccinated.
- HPV can be administered in a 3-dose series to individuals beginning at age 9 years.
Figures
Figure 1. Recommended Immunization Schedule for Children with HIV Infection Aged 0 through 18 Years; United States, 2019

Download Guidelines
-
Section Only PDF (364.25 KB)
-
Full Guideline PDF (5.2 MB)
-
Recommendations Only PDF (367.5 KB)
-
Tables Only PDF (991.66 KB)
-
Dosing Tables PDF (644.33 KB)
